Maku J. O. , Gbadamosi A. E. , Fadoju O. A.
Department of Plant Science and Biotechnology, Adekunle Ajasin University, Akungba-Akoko, Ondo State, Nigeria
Correspondence to: Maku J. O. , Department of Plant Science and Biotechnology, Adekunle Ajasin University, Akungba-Akoko, Ondo State, Nigeria.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Abstract
Wood is an important forest produce which play an important role in the life of men from cradle to the grave, Mansonia altissima is a good example of these forest produce. The growth of M. altissima (A. Chev) under different growth hormone was investigated. Seeds were collected from Ondo State Agricultural Development Project (ADP) Akure (Lat. 07015'N and Long 05005'E) and was raised in the nursery. Two hundred and ten (210) uniformly growing seedlings were transplanted into polythene bags and after two weeks of acclimatization, seedlings were laid out in the open nursery in a completely randomized design. Metric characters of the seedlings were taken fortnightly, biomass assessment, net assimilation rate and relative growth rate was done at the end of the experiment. Seeds treated with 0.03g/l IAA and 0.005g/l IBA germinated four days after sowing (DAS), those treated with 0.015g/l IAA, 0.005g/l IBA, 0.01g/l GA, 0.01and 0.02g/l NAA germinated 5DAS while seeds treated with 0.015g/l NAA were the last batch to germinate (8DAS). The highest germination percentage of 66 was recorded in seeds soaked in 0.02g/l IAA while the lowest germination rate of 38% was recorded in 0.005g/l IBA. There were significant (p≤0.05) differences in height of seedlings among the seedlings subjected to different growth hormone. The value for the biomass assessment was recorded at the end of the study. The net assimilation rate (NAR) and relative growth rate (RGR) were also recorded. The study indicated that the germination of seeds and growth of Mansonia altissima seedling responded well to the treatments of plant growth factors/ growth hormones and this could be adopted in raising this species for mass plantation establishment purposes.
Keywords:
Seed germination. Early seedling growth, Biomass assessment, Plant growth factors, Mansonia altissima
Cite this paper: Maku J. O. , Gbadamosi A. E. , Fadoju O. A. , Germination and Seedling Growth of Mansonia altissima (A. Chev) in Response to Hormonal Treatment, International Journal of Agriculture and Forestry, Vol. 4 No. 4, 2014, pp. 269-274. doi: 10.5923/j.ijaf.20140404.02.
1. Introduction
The tropical rainforest is a dense and luxuriant vegetation type and are the most biologically diverse terrestrial ecosystem [1]. In Nigeria, the tropical rainforest consist of trees seldom more than 60m high (200 feet tall), 2 – 3 feet (6 – 10m) trunk diameter [2]. The tropical rainforest in Nigeria contains some general utility species; these include Mansonia altissima, Milicia excelsa and Parkia biglobosa among others.M. altissima belongs to the family Sterculiaceae [3]. It is a semi – deciduous forest species growing up to 37m high, trunk to 2⅟2m girth, cylindrical to tapering with plank buttresses up to 2⅔m, bearing a dense crown, deciduous in the dry season [3]. Mansonia is classified as a non – pioneer light demander [4]. However, many of the tropical species are faced with many problems including annual bush burning, over exploitation and seed dormancy [5]; and they face the dangers of extinction from human induced deforestation [6].Human have being exploiting forest from time immemorial as they need to use forest species as sources of food, fuel and timber; or to clear forested land for agricultural and urban purposes [7]. On a global scale, world forests are declining at an extreme alarming rate due to increase in human population growth, which has doubled in the last 50 years. From 1990 to 2005, the world lost 30% of its total area of forested land, an average decrease of some 0.2% per year [1].In an attempt to mitigate the human impact on the forest biological structure and replace cut stands, seeds which have germination difficulty can be subjected to germination enhancing treatments such as acids, cold or hot water and hormonal preparation [8]. The categories of plant growth factors associated with germination and seedling growth / development include gibberellic acid (GA3), indole acetic acid (IAA), naphthalene acetic acid (NAA) and indole butyric acid (IBA) [8].M. altissima has been described as vulnerable according to the IUCN Red list of threatened species [9]; it therefore requires urgent conservation attention. It has been observed and documented that most of the timber tree species have difficulty regenerating them through seeds. [10], noted that there are many gaps in our understanding of tropical seeds, of which seed pre-treatment is one. The growing demand for seed banks for reforestation programs underlines the need for better theoretical and practical knowledge of the behaviour of such seeds. Therefore, this study aims at finding means of accelerating germination and enhancing seedling growth of M. altissima using plant growth factors.
2. Materials and Methods
Seedlings were raised from matured seeds collected from Agricultural Development Project (ADP) Akure (Lat. 07015'N and Long 05005'E) and raised at the green house of Adekunle Ajasin University, Akungba-Akoko (Lat. 70 27'N and long. 50 44'E: 390m above sea level). Twenty – one pre-treatment methods involving four plant growth factors; indole acetic acid (IAA), indole butyric acid (IBA), naphthalene acetic acid (NAA) and gibberellic acid (GA3) at five concentration levels of 0.005g/l, 0.01g/l, 0.015g/l, 0.02g/l, and 0.03g/l including control were tested. 50 seeds were allocated to each treatment; seeds were soaked for 24hrs and later air – dried for 48hrs before sowing in washed river sand. At the two leaf stage, 210 uniformly growing seedlings were transplanted into polythene bags filled with top soil. These were allowed to wean (acclimatize) for three weeks and later transferred to the open nursery in a completely randomized design.Seedlings morphological traits; the height, collar diameter, and number of leaves were measured fortnightly for 12weeks. Height of the seedlings were measured from the collar to the tip of the apical bud using a ruler calibrated in centimetre (cm), diameter readings were taken using a vernier caliper while the number of leaves of each seedlings were counted and recorded.Five seedlings were randomly selected under each hormonal treatment at the end of the experimental period (3 months) for biomass assessment. These seedlings were uprooted and the soil around the root was carefully washed off. The up rooted components were therefore separated into root, stem, and leaf component. The leaf area of seedlings was determined by tracing each leaf on a graph sheet using the proportional method of [11]. The root, shoot, and leaf components of each seedling were put into separate envelopes for ease of identification. Each envelope was weighed using an electronic weighing balance to determine the fresh weight of the samples after which the envelopes together with their contents were oven dried for 24h at 80℃. The samples were reweighed to get the dry weights. The data obtained were subjected to ANOVA, using SPSS and means were separated using Duncan’s Multiple Range Test (DMRT) at 5% level of significance.
3. Results
3.1. Cumulative Germination Percentage
Seedling emergence was first observed four (4) days after sowing (DAS) in seeds treated with 0.03g/l IAA and 0.05g/l IBA, followed by five days after sowing with seeds treated with 0.15g/l IAA, 0.005g/l IBA, 0.01g/l GA and 0.01 and 0.02g/l NAA while seeds under 0.015g/l NAA were the last batch to germinate eight day after sowing (DAS). The highest germination percentage of 66% was recorded in seeds soaked in 0.02g/l IAA, followed by seeds soaked in 0.01g/l IAA, 0.03g/l IAA and 0.02g/l IBA which had cumulative germination of 62% while the lowest germination of 38% was recorded in 0.005g/l IBA. (Figure 1).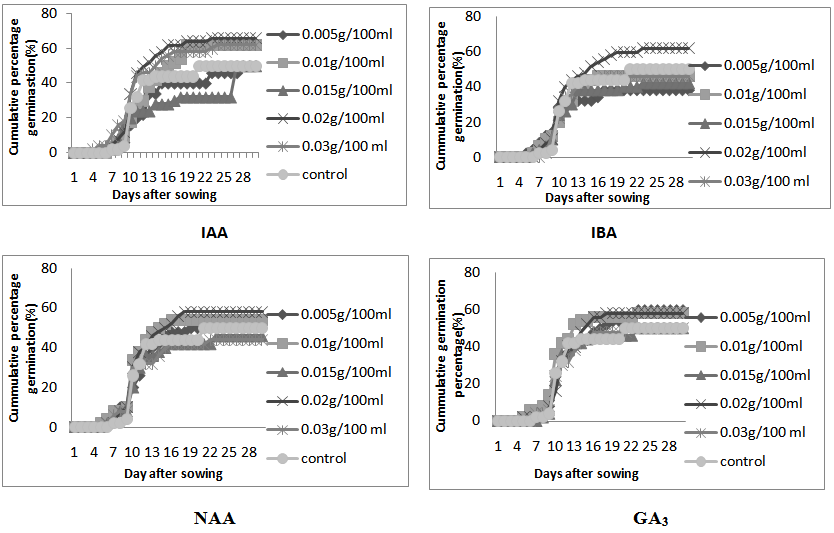 | Figure 1. Cumulative germination percentage if M. altissima seeds subjected to IAA, IBA, NAA, and GA3 respectively |
3.2. Seedlings Metrical Traits
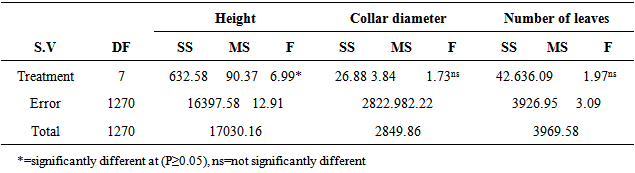
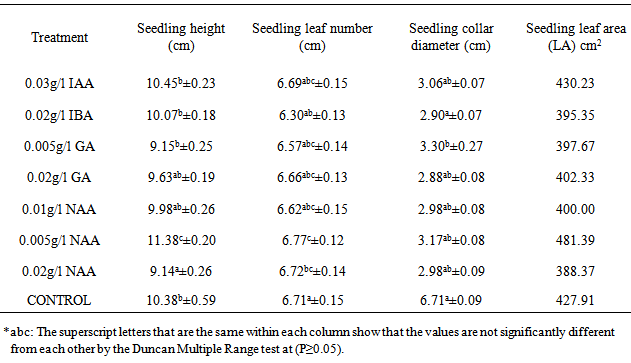
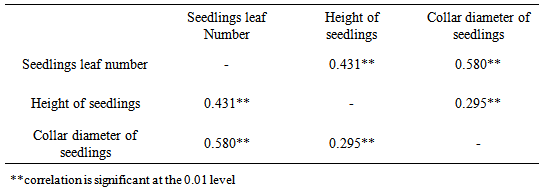
The effect of plant growth factors was significant (P≤0.05) on height of the seedlings of M. altissima (Table 1). The seedlings under 0.005g/l NAA had the highest mean seedling height value of 11.38cm ± 0.20; this was followed by seedlings under 0.03g/l IAA with a mean value of 10.45cm ± 0.23 while seedlings subjected to 0.02g/l NAA had the lowest mean value of 9.14cm ± 0.26. The mean height of seedlings under 0.01g/l NAA and 0.02g/l GA were significantly different from the mean height of seedlings under other treatment (Table 2). Height of seedlings is positively correlated with number of leaves (0.431) and collar diameter (0.295) of their seedlings (Table 5).There were significant (P≤0.05) differences in the number of leaves among the seedlings of M. altissima under different hormonal treatments (Table 1). The seedlings under 0.01g/l NAA had the highest mean value of 6.77cm ± 0.12; this was followed by seedlings under 0.03g/l IAA with a mean value of 6.69cm ± 0.15 while seedlings subjected to 0.02g/l IBA had the lowest mean value of 6.30cm ± 0.13. The mean number of leaf of seedling under 0.01g/l NAA was significantly different from the mean of seedlings under other treatments (Table 2). The seedling leaf number is positively correlated with height of the seedlings (0.431) and collar diameter of the seedlings (0.580) (Table 5).The seedlings under 0.01g/l NAA had the highest mean value of 11.38cm ± 0.02, followed by seedlings under 0.03g/l IAA with a mean value of 10.45cm ± 0.23 while seedlings subjected to 0.02g/l NAA had the lowest mean value of 9.14cm ± 0.26. The mean height of seedlings under 0.1g/l NAA was significantly different from the mean height of seedlings under other treatments (Table 2). Height of seedlings is positively correlated with number of leaves (0.431) and collar diameter (0.295) of the seedlings (Table 5).There were no significant (P≥0.05) differences in collar diameter of M. altissima subjected to different hormonal treatments (Table 1). The seedlings under 0.005g/l GA3 had the highest mean value of 3.30cm ± 0.27; this was followed by seedlings under 0.1g/l NAA with a mean value of 3.17cm ± 0.08 while seedlings under control treatment had the lowest mean value of 2.82cm ± 0.09. The mean collar diameter values of seedlings under hormonal treatment were not significantly different from each other (Table 2). Collar diameter of the seedlings is positively correlated with height of the seedlings (0.295) and leaf number of seedling (0.580) (Table 5).The seedlings of M. altissima under 0.005g/l NAA had the largest leaf area (LA) cm2 with a value of 481.39cm2, this was followed by seedlings under 0.03g/l IAA with a value of 430.23cm2 while seedlings under 0.02g/l NAA had the smallest leaf area of 388.37cm2 (Table 2).Table 1. Analysis of variance (ANOVA) for Metrical traits in seedlings of M. altissima subjected to different hormonal treatments
 |
| |
|
Table 2. Mean values of seedlings metrical traits and Leaf area of M. altissima subjected to different hormonal treatment
 |
| |
|
3.3. Seedling Biomass
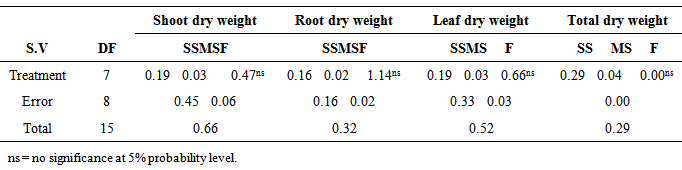
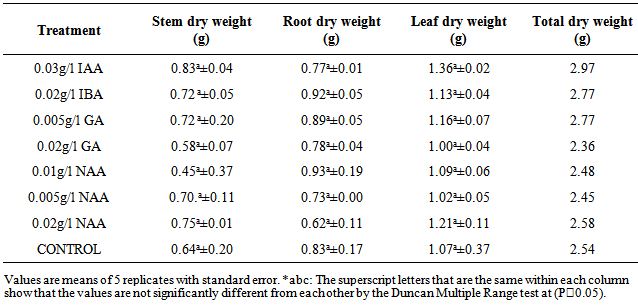
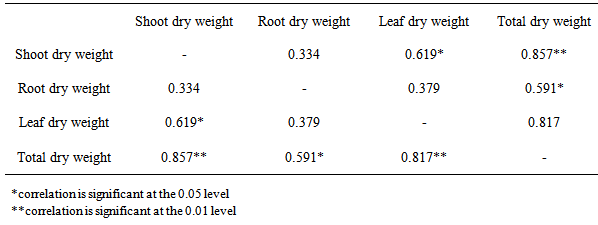
There were no significant (P≥0.05) differences in the stem dry weight of M. altissima subjected to different hormonal treatments (Table 3). The highest mean value of 0.83cm ± 0.04 was recorded in 0.03g/l IAA; this was followed by stem dry weight under 0.02g/l NAA with a mean value of 0.75cm ± 0.01 while the lowest mean value of 0.64cm ± 0.20 was recorded in control. The mean stem dry weight of M. altissima was not significantly different from each other (Table 4). Stem dry weight is positively correlated with RDW (0.334), LDW (0.619), and TDW (0.857) of the seedlings (Table 6).There were no significant (p≥0.05) differences in the root dry weight of M. altissima subjected to different hormonal treatments (Table 3). The highest mean value of 0.93cm ± 0.19 was recorded in 0.01g/l NAA; this was followed by root dry weight under 0.02g/l IBA with a mean value of 0.92cm ± 0.05 while the lowest mean value of 0.62cm ± 0.11 was recorded in 0.02g/l NAA. The mean root dry weight value of M. altissima was not significantly different from each other (Table 4). The root dry weight is positively correlated with SDW (0.334), LDW (0.379), and TDW (0.591) of the seedlings. (Table 6)There were no significant (p≥0.05) differences in the leaf dry weight of M. altissima subjected to different hormonal treatment (Table 3). The highest mean value of 1.36cm ± 0.02 was recorded in 0.03g/l IAA; this was followed by leaf dry weight under 0.02g/l NAA with a mean value of 1.21cm ± 0.11 while the lowest mean value of 1.00cm ± 0.04 was recorded in 0.02g/l GA. The leaf dry weight mean value of M. altissima was not significantly different from each other (Table 4). The leaf dry weight is positively correlated with SDW (0.619), RDW (0.379), and TDW (0.817) of the seedlings. (Table 6)There were no significant (p≤0.05) differences in the total dry weight of M. altissima subjected to different hormonal treatment (Table 3). The seedlings under 0.03g/l IAA had the highest total dry weight with mean value of 2.97g; this was followed by seedlings under 0.02g/l IBA and 0.005g/l GA while the lowest mean value of 2.36g was recorded in seed soaked in 0.02g/l GA (Table 4). The total dry weight is positively correlated with SDW (0.857), RDW (0.591), and LDW (0.817) of the seedlings. (Table 6)Table 3. ANOVA for Biomass production in seedlings of M. altissima subjected to different hormonal treatment
 |
| |
|
Table 4. Mean values for seedlings biomass of M. altissima subjected to different hormonal treatment
 |
| |
|
Table 5. Correlation Analysis of Metric characters of M. altissima
 |
| |
|
Table 6. Correlation Analysis of Biomass parameters of M. altissima
 |
| |
|
4. Discussion
Plant growth factors / plant growth hormones are known to stimulate seed germination; this has been documented by various authors ([12]; [13]). The effect may be explained with indole acetic acid (IAA) which had the highest germination percentage and according to Fletcher et al, (2000); treating seeds with growth regulators is known to influence plant growth and stress resistance. Two important parameters affecting growth regulator penetration into seeds are the concentration of growth regulator and duration of treatment [14]. The nature of the solvent, in which growth regulators are delivered also influence the rate and amount of growth regulator penetration into seeds according to [15] suggested that treating seeds with growth regulators contribute to inefficient energy metabolism, lowering indole – 3 acetic acid levels in treated seeds could be a side effect of GA3 inhibition since GA3 induces IAA biosynthesis [16] and that, some unwanted effects from treating seeds with growth regulators include reduction and delay in seedling emergence ([17] and [14]). Seed treatment with plant growth factors (hormones) increase seedling height of M. altissima, which is in agreement with the findings of [18] that IAA, IBA at low concentrations enhanced seedling height, collar diameter and number of leaves of Tetrapleura tetraptera. However, the results showed that there were differences in height of M. altissima; the seedlings subjected to naphthalene acetic acid (NAA) had a higher mean value than those of Indole acetic acid (IAA), indole butyric acid (IBA), gibberellic acid (GA3) and control. This is due to the fact that NAA promote cell division and expansion and helps to induce the formation of adventitious roots. Results showed that the collar diameter of M. altissima seedlings was increased by the application of plant growth regulator compared to control. This increase may be due to interaction between gibberellic and endogenous auxin which might have promoted xylem production and cambium growth. Results from work with woody species has indicated that auxin (IAA, IBA and NAA), rather than GA3, was the predominant factor regulating cambium formation, notwithstanding, in the presence of auxin and enhanced GA3 production gives some stimulation of cambium proliferation [19]. Well-formed cambium is a good property of tree species for timber and wood purposes.The enhanced CDT because of hormonal treatment will hasten the return on investment as a timber species since plant growth factors hasten the growth of Mansonia. Furthermore, the study showed that even under hormonal concentration, the seedlings of M. altissima were significantly different in their leaf number which concur with [12] findings which found that growth regulator enhance activity of enzymes and thus protect the vegetative structure of plants from a variety of stress, higher temperature and photo inhibition; and some activities [20]. The results indicated that auxins – IAA, IBA, and NAA increased the growth parameters of the plants resulting in increased overall development as compared to control. Lower concentrations, 0.005g/l, and 0.01g/l achieved significant increase in seedlings spread and seedlings volume over control. Also, all plant growth regulators showed significant effect in seed germination and the seedling growth of M. altissima plants marked by the onset of reproductive phase when compared to control.Net assimilation rate (NAR) is more closely correlated with changes in relative growth rate (RGR) in M. altissima. According to [21], significant correlations were found on the effects of temperature in plant growth which are likely to be partly mediated through its effect on photosynthesis and respiration of plant adopted to resource – limiting habitat lower than their counterparts from resource – rich environment when grown under identical controlled conditions in the presence of adequate water and nutrients. In addition, the correlation analysis on biomass assessments of M. altissima was significant at the 0.05 level and 0.01 levels.
5. Conclusions
Considering the results gotten from this study and the effects of the treatments on the germination and the morpho-physiological development of M. altissima, it could be concluded that auxins at lower concentration (i.e. 0.005g/l and 0.01g/l NAA, IBA and IAA) should be adopted in raising the seedlings of M. altissima for plantation establishment.
ACKNOWLEDGMENTS
I want to use this opportunity to appreciate Mr O.S. Osekita, Mr A.T. Ajayi and Mr. D. Olatunji in the Department of Plant Science and Biotechnology, Adekunle Ajasin University Akungba Akoko Nigeria for the editing, data analysis, and other technical supports rendered during this study.
References
| [1] | FAO 2007. State of the World’s Forests 2005. FAO, Rome, Italy. |
| [2] | Nelson R.W. 1996, Natural forest disturbance and change in the Brazilian Amazon. Remote Sensing Reviews 10: 105–125. |
| [3] | Irvine, F.A.R. 1961, Woody plants of Ghana with special reference to their uses. Oxford Univ. Press London p 146. |
| [4] | Gyimah, R., T.Nakao and Min 2003, Effect of light intensity and nutrient on growth and electron transport of tropical tree found in Ghana, Faculty of Agriculture, Miyazaki University, Japan. 40pp. |
| [5] | Beet W.C. 1989, The potential role of agro forestry in ACP countries. Technical centre of Agric and Rural Cooperation (ACP-SEC IOME CONVENTION) Netherland. |
| [6] | Myers, N, 2000, Deforestation Rates in Tropical Forests and their Climatic implications. Friends of the Earth, London. |
| [7] | Young, A.G and Boyle, T.J. 2000, Forest fragmentation. In Young A, Boshier, D. and Boyle, T. (eds.), Forest conservation genetics: principles and practice. CSIRO Publishing, Collingwood. |
| [8] | Agboola, D.A, and Adedire M.A. 1998, Responses of germination promoters. Nigeria Journal of Botany 11: 103 - 110. |
| [9] | IUCN (The world conservation Union) 2008, 2008 Red list of threatened species. |
| [10] | Bonner, F.T. 1990, Storage of seeds: potential and limitations for germplasm conservation. Forest Ecology and Management 35:35-43. |
| [11] | Eze J. M.O. 1965, Phytoremediation of heavy metals by Erichbornia crassipes. Journal of Earth and Environmental Science 27: 349-355. |
| [12] | Fletcher, A., A. Gilley, N. Sankhla, and T. Davies. 2000, Triazoles as plant growthregulators and stress protectants. Hort. Rev. 24: 55-138. |
| [13] | Rademacher, W. 2000, Growth retardants: effects on gibberellin biosynthesis and other metabolic pathways. Ann. Rev. Plant Physiol. Plant Molec. Biol. 51: 501-531. |
| [14] | Pill, W. G. and J. A. Gunter. 2001, Emergence and shoot growth of cosmos and marigold from paclobutrazol-treated seed. J. Environ. Hort. 19: 11-14. |
| [15] | Bai, S. and W. Chaney. 2001, Gibberellin synthesis inhibitors affect electron transport in plant mitochondria. Plant Growth Reg. 35: 257-262. |
| [16] | Koukourikou-Petridou M. A. 1996, Paclobutrazol affects growth of almond fruits and germination of almond seeds. Plant Growth Reg. 20: 267-269. |
| [17] | Giba, Z., D. Grubisic, and R. Konjevic. 1993, The effect of white light, growth regulators and temperature on the germination of blueberry (Vaccinium myrtilis L.) seeds. Seed Sci. Technol. 21: 521-529. |
| [18] | Maku J.O, Gbadamosi A.E, Oke S.A. 2014, Effect of some growth hormones on seed germination and seedling growth of Tetrapleura tetraptera (Thaub). International Journal of Plant Research.Vol.4 (1). Pp 36-42. |
| [19] | Elo A., Immanen J., Nieminen K., Helariutta Y. 2009, Stem cell function during plant vascular development. Semin. Cell Dev. Biol. 20: 1097–1106. |
| [20] | Beltagi, M. S., A. A. H. Saleh, and E.-S. A. El-Meleigy. 2002, Effect of cadmium and paclobutrazol on anabolic capacities and electrophoretic protein patterns in peanut (Arachis hypogeae) plant. Pakist. J. Biol. Sci. 5: 196-200. |
| [21] | Veeneedeer J, de la Cerda I.G, Boucher D, Perfecto I & Ruiz J.2000, Hurricane disturbance and tropical tree species diversity. Science 290: 788–791. |


 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML




