-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2014; 4(3): 246-254
doi:10.5923/j.ijaf.20140403.17
Status and Diversity of the Cassava Mosaic Disease Causal Agents in Sierra Leone
Alusaine E. Samura1, Festus B. Massaquoi1, Augustine Mansaray1, P. Lava Kumar2, J. P. C. Koroma3, S. N. Fomba1, A. G. O. Dixon4
1Natural Resource Management, Njala Agricultural Research Center (NARC)
2International Institute of Tropical Agriculture (IITA), PMB 5320, Ibadan, Nigeria
3Department of crop protection, Njala University
4Sierra Leone Agricultural Research Institute (SLARI), Freetown, PMB 1313, Sierra Leone
Correspondence to: Alusaine E. Samura, Natural Resource Management, Njala Agricultural Research Center (NARC).
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Cassava is the most important root and tuber crop in Sierra Leone. Its low yield can be attributed to several production-limiting factors including cassava mosaic disease (CMD). This study examined in a much wider scope the diversity, prevalence, distribution, incidence and level of severity of the CMD within the cassava farming communities across major agro ecologies in Sierra Leone. A survey was conducted October, 2010. Field assessment was also conducted on farms evaluated. Data was collected on the spot and complimented with group discussions and interviews. Field coordinates were determined using a global positioning system (GPS) recorder. This study showed a countrywide prevalence of 85.2% out of 156 sites visited using GPS mapping. The rain forest ecology had the highest prevalence of 97.2% while the coastal plain had the lowest disease prevalence. Incidence of CMD per district was generally high. Tonkolili district recorded the highest incidence of 99.2% followed by Kailahum and Pujehun. Bonthe district had the lowest severity score, while pujehun district had the highest severity score. Difference in CMD infection was also observed in terms of agro-ecology. Test using polymerase chain reaction (PCR) detected African cassava mosaic virus (ACMV) and also for the first time the East African cassava mosaic virus (EACMV) in two locations in the Moyamba district, southern Sierra Leone. The result from this study indicates the need for an increased adoption of CMD resistant cassava genotypes that are high yielding, has good cooking quality and with the ability to replace the local choice variety without significantly altering the cultural and aesthetic quality of the generally accepted local cultivar.
Keywords: Incidence, Severity, Diversity, Cassava mosaic geminivirus
Cite this paper: Alusaine E. Samura, Festus B. Massaquoi, Augustine Mansaray, P. Lava Kumar, J. P. C. Koroma, S. N. Fomba, A. G. O. Dixon, Status and Diversity of the Cassava Mosaic Disease Causal Agents in Sierra Leone, International Journal of Agriculture and Forestry, Vol. 4 No. 3, 2014, pp. 246-254. doi: 10.5923/j.ijaf.20140403.17.
Article Outline
1. Introduction
- Cassava (Manihot esculenta Crantz) is the second most important food crop after rice in Sierra Leone; it is also the most important root and tuber crop [21]. Cassava is also grown all over the country which has shown remarkable success in its processing at both domestic and commercial scales. Cassava was introduced to Africa by the Portuguese during the late 16th century [18]. It was readily adaptable to the different environmental conditions and well suited for integration into the farming systems and socioeconomic conditions of Africa. In Sierra Leone, cassava is processed into some common products: gari, lafun, starch and boiled cassava with beans. Cassava-based products such as raw tubers, gari and cassava bread (very thin, small, flat, round pieces) are traded mainly in Sierra Leon [14]. Cassava leaves provide source of income for women. The leaves are used to prepare a very popular national cassava leaf sauce [20].Average yield of cassava in Sierra Leone is low and is estimated at 7.18 t/ha according to FAO 2012. [8]. The low yields of cassava in Sierra Leone can be attributed to several production-limiting factors. These include, among others diseases, pests and weed infestation, as well as edaphic, agronomic and socio economic factors [1]. Diseases have been shown to play a prominent role in limiting cassava productivity in Sierra Leone. Some of those of economic importance include the cassava mosaic disease (CMD), cassava bacterial blight (CBB), and recently cassava brown streak virus [5].The CMD is endemic to Africa, caused by at least 7 different species of whitefly (Bemisia tabaci) transmitted geminiviruses, commonly referred as cassava mosaic geminiviruses (CMGVs) [16]. They are likely descendants of geminiviruses adapted to infect indigenous uncultivated African plant species [9]. Therefore the adaptation of CMGV’s to cassava could have only commenced, either after cassava was introduced to West Africa in the Gulf of Guinea during the 16th century, or after it was introduced to East Africa and the SWIO islands in the 18th century [9], [13]. Since the initial characterization in the early 1980s of the “first” CMG species, African cassava mosaic virus (ACMV) [3], it has subsequently been discovered that African CMG’s in fact consist of at least seven distinct begomovirus species including South African cassava mosaic virus (SACMV), East African cassava mosaic virus (EACMV), East African cassava mosaic Cameroon virus (EACMCV), East African cassava mosaic Zanzibar virus (EACMZV), East African cassava mosaic Malawi virus (EACMMV), and East African cassava mosaic Kenya virus (EACMKV) [4], [15]. Recently, two species have also been newly described: African cassava mosaic Burkina Faso virus (ACMBFV) and Cassava mosaic Madagascar virus [23]Several species and strains of cassava mosaic geminivirus have been described [10]. Limited information on cassava mosaic viruses have been provided from Sierra Leone in terms their distribution and none on their effects on growth or yield. These are serious deficiencies and thus emphasize the inadequate attention given to the cassava mosaic viruses in Sierra Leone which affect the most important root and tuber crop. The traditional belief that mosaic infected cassava genotypes act an indicator for the identification of poundable cassava genotypes with high dry matter content has encouraged the use of locally infected genotypes. Further there is a popular demand for mild mosaic infected cassava leaves among women for preparing a popular vegetable sauce using cassava leaves. It is believed that the infected leaves due to the low chlorophyll content consumes less palm oil, an expensive but crucial element in the preparation of the cassava leaf sauce compared to improved resistant varieties which consumes more. Women play a vital role in the selection of planting materials and would influence varieties of their choice which in most cases would include infected local varieties for domestic consumption. Results of preliminary surveys carried out by [22] indicated that ACMV is prevalent throughout the western areas of Sierra Leone with an estimated 100% incidence in smallholder farms. On the spot check on farmers’ field in other part of the country such as Bo, Kemena, Pujehun, and Njala indicate similar trends which warrant a nationwide survey on the prevalence and distribution of the mosaic virus as well as the diversity of the CMGVs.The large molecular diversity with viruses from east Africa points to the region as the centre of diversification [10]. In addition and more significantly recombination evident in a number of virus genomes is a driving force of geminivirus evolution. Virus diversity and frequent recombination events found in virus genomes provide evidence for continuous evolutionary processes and influence the development of epidemics and the emergence of ''new'' viruses. The knowledge of virus diversity, the geographic distribution of virus types and the structure of virus populations is a significant prerequisite to deploy cassava with virus resistance characters. This study examined in a much wider scope the different CMGVs and their distribution within the cassava farming community in Sierra Leone. Elucidation of the current prevalence and distribution of species/strains of the CMGVs will provide new opportunities for developing effective interventions. These include developing cassava genotypes with appropriate source of resistance as well as farmer’ desired traits for the prevailing environment and subsequently increase yields and productivity of cassava. The study was therefore undertaken with the following objectives in mind:1. To identify diversity among cassava mosaic geminivirus group within Sierra Leone 2. To determine prevalence, incidence, severity and distribution of CMV in Sierra Leone
2. Materials and Method
2.1. Survey Routes
- The survey was conducted between October and November 2010. The survey routes were determines using the road maps of Sierra Leone and such routes includes highways, secondary roads and feeder roads. The route was selected to target major cassava growing area within the geo political districts as well as the major agro ecologies in Sierra Lone.
2.2. Field Assessment
- Sample sites were selected along the route based on diversity of the villages as well as farms in order to capture diversity of the cassava mosaic virus. Approximate age of the crop/plants (in months), variety, origin of stem cuttings, and duration of the cultivar was investigated. Group discussion and interviews was conducted to compliment field data. This ranged between 2-3 km in smaller chiefdoms and 3-5 km in bigger chiefdoms. A total of 156 fields were visited. In each field, the coordinates were recorded using the Global positioning system (GPS) GARMIN e Trex Legend 1200 E 151st Street, Olathe, Kansaa 66062 U.S.A).
2.3. Disease Assessment
- On the spot assessment was conducted in all fields in the western Area and 12 districts and 5 agro- ecologies which include the rain forest, savannah lowland, savannah highland, coastal plains and the peninsular mountain. Prevalence was calculated as the number of site infected over the total number of sites visited expressed in percentages. Leaf sample was collected from the dominant variety grown and from other plants showing distinct diversity. Incidence and severity of the cassava mosaic virus was assessed using the five point scale adopted by [12] as follows1 = Symptom-less plants 2 = Mild chlorotic patterns affecting most leaves; mild distortions at the bases of most leaves and remaining part of the leaves are normal3 = Pronounced chlorosis on most leaves, narrowing and distortion of the lower one-third of the leaflets. 4 = Severe chlorosis and distortion of two-thirds of most leaves and general reduction of leaf size and some stunting. 5 = Most severe symptoms (severe chlorosis, leaf distortion, twisting, misshapen leaves, severe reduction of most leaves and severe plant stunting)Percent incidence was calculated by expressing in percent the total number of infected plant over the total number of plants sampled. 5 Leaf sample was collected from each field for diverse symptom types; severe, moderate, mild, no symptoms).The field with an overall impression of symptom severity score 2 was regarded as mild, score 3 as moderately severe and 4 or 5 as severe. Infection type was determined as, A) Cutting-borne: Where all leaves of the infected plants show uniform symptoms.B) Whitefly-borne: Where symptoms may not be apparent on old leaves (bottom portion of the plant), but clear symptoms on newly produced leaves (top portion of the plant).C) Super infection: Where different symptoms in the same plant. Old leaves (bottom portion) show mild or moderate symptoms; and newly emerging leaves (top portion) show severe symptoms (symptoms very different from the lower portion).Adult whitefly was counted from the lower side of the leaf on the five topmost fully-formed apical leaves of the tallest shoot on ten randomly selected plants diagonal to the farm’s orientation. Leaf sample was collected based on the diversity of symptom expression as described above. In transit leaf sample was stored in 4℃ refrigerator prior to analysis at the Virology and Molecular and Diagnostic Unit at the International Institute of Tropical Agriculture (IITA) for analysis.
2.4. Laboratory Analysis of Samples
2.4.1. DNA Extraction
- Total DNA was extracted from eighty cassava leaf samples according to the procedures of [6]. Extracted DNA from leaf sample was resuspended in 200µl TE (Tris- HCL 50mM, EDTA 10mM) and p H 8.0 and stored at -20℃.
2.4.2. Testing of DNA Samples
- Leaf samples were tested by polymerase chain reaction (PCR). DNA from leaf samples was diluted to obtain 2 ng/µl. The specific primers for the detection of ACMV, EACMV, ACMV + ECMV and EACMV – UG was used as represented in Table 1. The reaction mixture per tube contained 2.5 µl of the Green buffer (10 x concentration), 0.75 µl MgCl2; 0.25 µl of dNTP, 0.25 µl each of forward and reverse primers, 0.06 µl of taq DNA polymerase (Promega, USA) 6.44 µl sterilized distilled water, and 2.2 µl of healthy cassava were used as negative control. The healthy negative control clone was obtained from virus tested plant. EACMV DNA (obtained from the virology and molecular diagnostic unit IITA Ibadan) was used as a positive control for the detection of the virus.
3. Result
3.1. Prevalence of the Cassava Mosaic Disease in Sierra Leone
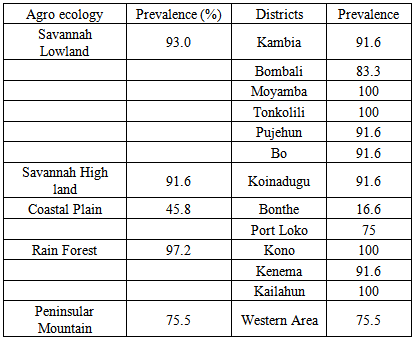
- Country wide the prevalence of cassava mosaic disease was 85.2% out of 156 sites visited. The Rain forest ecology comprising Kono, Kenema and Kailahum had the highest prevalence of 97.2% while the coastal plain had the lowest disease prevalence of 45.8%. On district bases, disease prevalence was high. Moyamba, Kono, Kailahun and Tonkolili districts had 100% prevalence of the disease while Bonthe district has considerably the lowest prevalence of 16.6%. (Table 2).
|
3.2. Incidence of CMD per District
- Incidence of CMD per district was generally high. Tonkolili, Moyamba, Kono, and Kailahum district recorded the highest incidence of 100.2% followed by Bo, Kambia Kidence enema, Koinadugu and Pujehun districts with 91.6% incidence, respectively. Bonthe district had lowest disease incidence of 16.67% followed by Port Loko and Western Area with 75% respectively (Table 3).
|
3.3. Severity of Cassava Mosaic per District
- Significant difference was observed within district in their response to CMD (P<0.05). Generally severity of CMD was mild based on the criteria adopted for severity ranking. Bonthe district had the lowest severity score of 1.26 which was considered to be resistant. Pujehun district had the highest severity score of 3.74 and was classified to be severe. Severity score of other districts ranged between 2.4 to 2.9 which were considered to be mild (Table 3).
3.4. The Distribution of Farms Showing Incidence and Severity of CMD in Sierra Leone
- CMD was widely distributed country wide with 112 (73.2%) of farms having an incidence of ranging between 81 to 100%. 26 (16.9%) of the farms surveyed had disease incidence of 20% and less (Fig 1).
 | Figure 1. Distribution of Farms Showing Incidence of cassava Mosaic Disease in Sierra Leone |
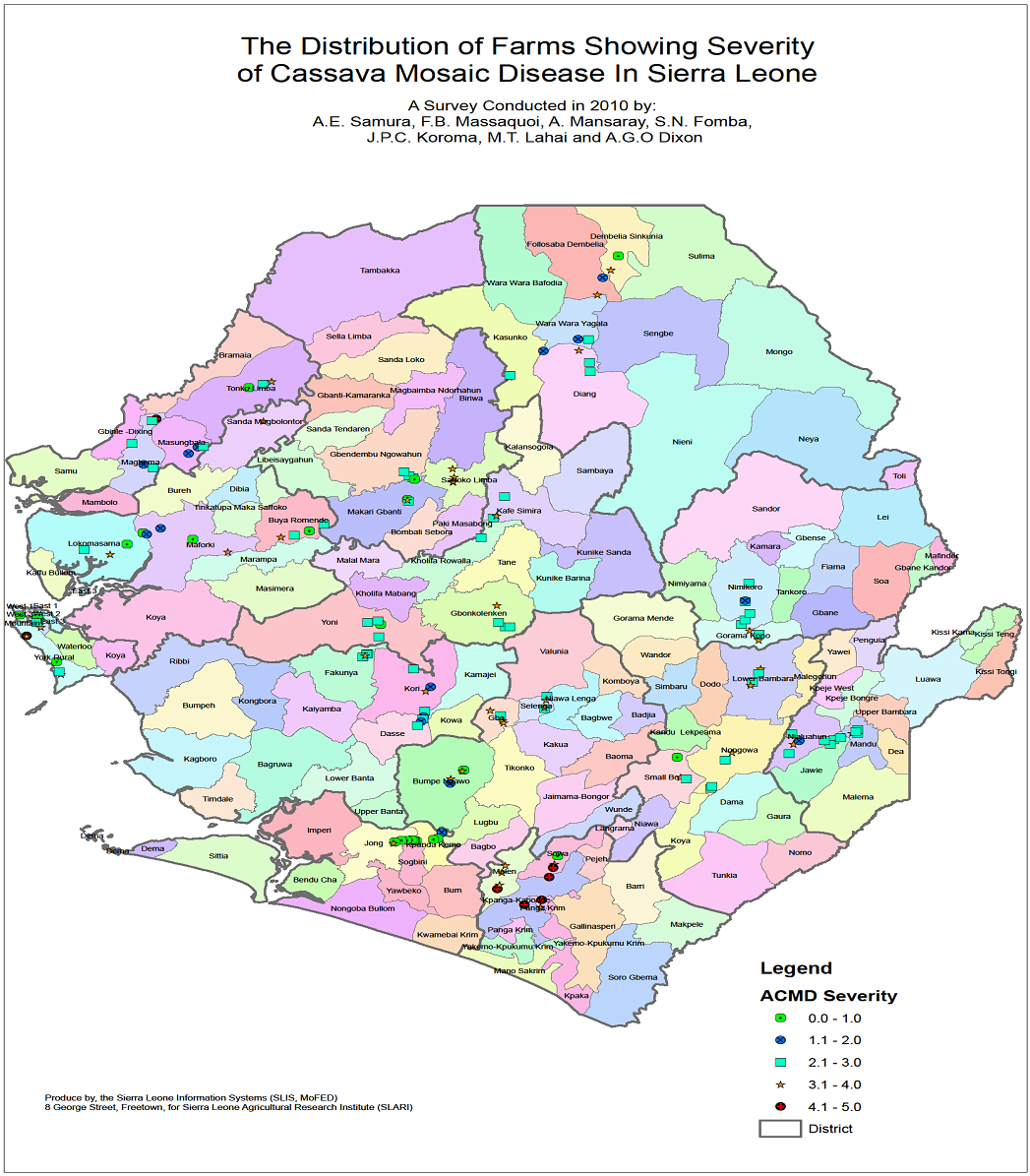 | Figure 2. Distribution of Farms Showing Severity of cassava Mosaic Disease in Sierra Leone |
3.5. CMD across Agro Climatic Ecologies in Sierra Leone
- Incidence and Severity of CMDThe Savannah lowland had the highest disease incidence of 89% followed by the Rain forest with 87.5%. The lowest disease incidence of 36.7% was observed in the coastal plain. In terms of disease severity, the rain forest zone had the highest severity score of 2.67 followed by the savannah lowland with 2.5. The coastal plain had the lowest severity score of 1.64. Based on the criteria adopted for resistance and susceptibility CMD severity was regarded as mild in all agro ecologies Table 4.
|
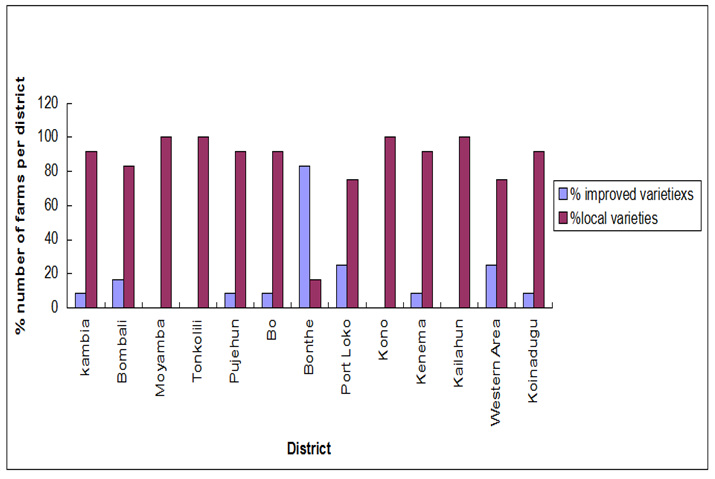 | Figure 3. Percent number of farms with improved and infected local varieties per district in 2010 |
3.6. Diversity of Cassava Mosaic Geminiviruses among Local Cassava Varieties in Farmer’s Field in Sierra Leone
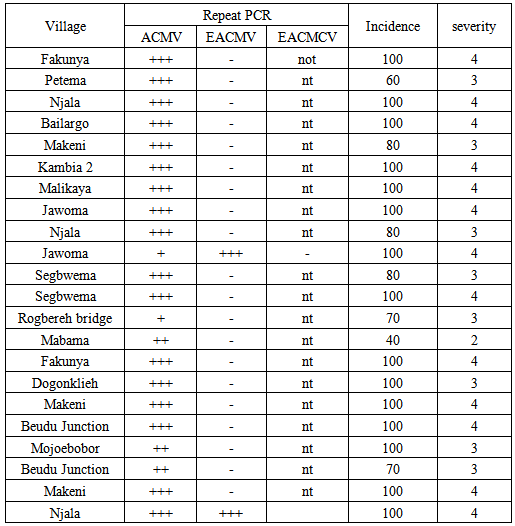
- Among the 80 leaf samples collected from symptom bearing plants, 70% tested negative to ACMV and EACMV. 28% of the samples tested positive for ACMV alone while 2% tested positive for EACMV.The most common cassava mosaic geminivirus found within cassava field from leaf samples was the African cassava mosaic virus (ACMV) which is dominant in West Africa. Out of eighty samples tested 20 were positive for ACMV infection alone while 2 samples at Jawoma and Njala tested positive for East African Cassava Mosaic Virus (EACMV) and ACMV. Symptom expression in the field did not always match PCR test. Severe symptom was expressed with EACMV infection and had a severity score of 4 while ACMV infection alone was comparatively lower with a score of 3.
|
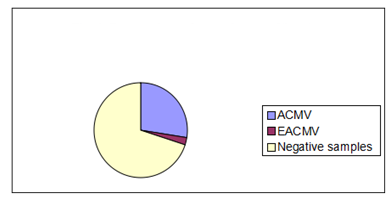 | Figure 4. Proportion of samples positive to cassava mosaic genimiviruses |
4. Discussion and Conclusions
- The high prevalence of CMD in Sierra Leone can be attributed to the widespread use of local varieties susceptible to the disease. In contrast however the Bonthe district which recorded a disease prevalence of 16.6% can be associated with the cultivation of a released variety SLicass 4 commonly referred to as blue boat. At the time of this survey, Puejun district had the highest disease severity score of of 3.74 and a prevalence rate of 91%. Since most of the infection is caused by the use of infected cutting breeding effort for control of the CMD should be geared towards replacing infected local varieties with farmer desired genotypes that are resistant to the disease. The adoption of Slicass 4 (Blue boat) in Bonthe is closely linked with high level of gari production. It can be concluded that the adoption of disease resistant varieties must be complimented by improved processing facilities. Efforts have been made to disseminate improved high yielding cassava genotypes which may have being captured during the survey as indicated in the incidence and severity map (Fig 1 & 2). This effort has to be compliment with functional processing facilities for meaningful impact to be in the control of the mosaic virus. Additionally the application tissue culture technique through in vitro multiplication of virus free plants for distribution to farmers may help reduce the prevalence rate of the disease as well as maintaining the genetic diversity of indigenous local varieties. It was widely believed that ACMV was the only species associated with cassava in Sierra Leone. This study provides information on the first report of the EACMV in Sierra Leone. In this survey only two samples tested positive for EACMV. Both samples were collected from the Moyamba district at Jawoma and Njala and were closest to the Njala Agricultural Research Center (NARC) which hosts the highest cassava germplasm collection in the country. This result is consistent with results observed in Nigeria where 0.05% of a total of 290 [17] and 0.3% out of a total of 1106 samples tested positive for EACMV. Plants with symptoms that tested negative to either ACMV and /or EACMV may be as a result of mistaken symptoms of cassava green mite which has a common symptom with CMD. However further studies are necessary to determine the virus diversity by sequencing for through knowledge on viruses in the country.
ACKNOWLEDGEMENTS
- The authors wish to extend profound thanks and gratitude to the Government of Sierra Leone, the West Africa Agricultural Productivity Project (WAAPP) and the International Institute of Tropical Agriculture (IITA) for finical and technical support to this project. Personnel from the molecular and diagnostic laboratory (IITA) are highly acknowledged.
References
| [1] | Alusaine E. Samura, Festus B. Massaquoi, Sahr N. Fomba, A. G. O. Dixon. 2013. Mitigating the Effect of Cassava Mosaic Disease (CMD) on Cassava Productivity in Sierra Leone: Past and Present Efforts. International Journal of Agriculture and Forestry 2013, 3(7): 353-361. |
| [2] | Berrie, L. C., Palmer, K. E., Rybicki, E. P. and Rey, M. E. C. (1998). Molecular characterisation of a distinct South African cassava infecting geminivirus. Archives of Virology 143:2253-2260. |
| [3] | Bock K, Woods R: Etiology of African cassava mosaic disease. Plant Dis 1983, 67:994-995. |
| [4] | Bull SE, Briddon RW, Sserubombwe WS, Ngugi K, Markham PG, Stanley J: Genetic diversity and phylogeography of cassava mosaic viruses in Kenya. J Gen Virol 2006, 87:3053-3065. |
| [5] | Calvert, L.A. and Thresh, J.M. 2002.The viruses and virus diseases of cassava. In Cassava biology, production and utilization, edited by A.C. Bellotti, R.J. Hillocks, and J.M. Thresh. CAB International, Wallingford, UK.332 pp. |
| [6] | Dellaporta, S. L. Wood J., Hicks, J. R. (1983). A plant DNA minipreparation: version II. Plant Molecular Biology Reporter 1:19-21. |
| [7] | Deng, D., P.F. McGrath, D.J. Robinson and B.D. Harrison, 1994.Detection and differentiation of whitefly- transmitted geminiviruses in plant and vector insects by the polymerase chain reaction with degenerate primers. Annals of applied Biology 125:327-336. |
| [8] | FAOSTAT, 2012. http:/faostat-gateway. |
| [9] | Fauquet C, Fargette D: African cassava mosaic virus: Etiology, Epidemiology, and Control. Plant Dis 1990, 74:404-411. |
| [10] | Fauquet, C. M, Bisaro, D. M., Briddon, R. W., Brown, J. K., Harrison, B. D., Rybicki, E. P., Stenger, D. C. and Stanley, J. (2003). Revision of taxonomic criteria for species demarcation in the family Geminiviridae, and an updated list of begomovirus species. Archives of Virology 148:405-421. Food and Agriculture Organisation (FAO), 2005. A review of cassava in Africa with country case studies on Nigeria, Ghana, The united Republic of Tanzania, Uganda and Benin. Vol 2. www.fao.org/doc rep/009/a0154e/ A0154E04.HTM. |
| [11] | FAOSTAT, 2012. http://faostat.fao.org/. |
| [12] | IITA (International Institute of Tropical Agriculture, (1990). Cassava in Tropical Africa, A reference Manual. IITA, Ibadan, Nigeria. 176pp. |
| [13] | Jones WO. 1959: Manioc in Africa. Stanford: Stanford University Food Research Institute; 1959.  |
| [14] | Latif O. Sanni, Oluwatobi O. Onadipe, Paul Ilona, M.D. Mussagy, A. Abass, and A.G.O. Dixon, 2009. Successes and challenges of cassava enterprises in West Africa: a case study of Nigeria, Bénin, and Sierra Leone. IITA, Ibadan, Nigeria. 19 pp. |
| [15] | Legg JP, Fauquet CM: Cassava mosaic geminiviruses in Africa. Plant Mol Biol 2004, 56:585-599. |
| [16] | Legg, J. P. and Thresh, J. M. (2003). Cassava virus diseases in Africa. In: Plant Virology in sub-Saharan Africa, 517 – 550pp. http://www.iita.org/info/virology.htm |
| [17] | Ogbe, F. O. (2001). Survey of cassava begomoviruses in Nigeria and the response of resistant cassava genotypes to African cassava mosaic begomivirus infection. Ph.D Thesis, Department of Crop Protection and Environmental Biology, University of Ibadn, Nigeria. Pp 197. |
| [18] | Olsen K, Schaal B. 1999. Evidence on the origin of cassava: Phylogeography of Manihot esculenta. Proc Natl Acad Sci USA, 96:5586-5591. |
| [19] | Pita, J. S., Fondong, V. N., Sangaré, A., Kokora, R. N. N. and Fauquet C.(2001). Genomic and biological diversity of the African cassava geminiviruses. Euphytica 120: 115-125. |
| [20] | S. N. Fomba, F. B. Massaquoi, A. E. Samura, D. S. Fornah, M. T. Benya, A. G. O. Dixon, A. Jallon, B. Alenkhe, O. Onadipe and L. O. Sanni. 2012. Innovative system to improve small to medium scale cassava processing in Sierra Leone. In |
| [21] | Sahr. N. Fomba, Festus B. Massaquou, Prince E. Norman, Alusaine. E. Samura, Augustine Mansaray, Abdulai Jalloh, Alfred. G. O. Dixon, Nyabeh A. Anthony, Micheal T. Benya., Daniel S. Fornah, Memuna K. Sawi, Alieu Sartie, Oluwatobi O. Onadipe and Latif O. Sanni, 2012. Current status of root and tuber crops improvement, production and utilization. In Proceedings of the 11th triennial symposium of the ISTRC-AB. |
| [22] | Thomas, M.D., 1999. Preliminary assessment of severity and incidence of African cassava Mosaic disease and its effect on yield in farmers field in Sierra Leone. Final report, University Research and Development Services (URDS) Bureau, University of Sierra Leone. |
| [23] | Tiendrébéogo F, Lefeuvre P, Hoareau M, Harimalala MA, De Bruyn A, Villemot J, Traoré VSE, Konaté G, Traoré AS, Barro N, Reynaud B, Traoré O, Lett JM. 2012.: Evolution of African cassava mosaic virus by recombination between bipartite and monopartite begomoviruses. Virol J, 9:67.  |
| [24] | Zhou, X.; Liu, Y.; Calvert, L.L; Muñoz, C.; Otim- Nape, G.W,; Robinson, DJ.; Harrison, B.D.1997. Evidence that DNA-A of a geminivirus associated with severe cassava mosaic disease in Uganda has arisen by interspecific recombination. J. Gen. Virol. 78:2101-2111. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML