-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2014; 4(3): 171-177
doi:10.5923/j.ijaf.20140403.04
Population Dynamics of the Hibiscus Mealybug Maconellicoccus hirsutus Green (Hom., Pseudococcidae) and Its Parasitoid on Guava Trees in Madaba-Jordan
Muna Al-Fwaeer 1, Ibtihal Abu-Obaid 2, Firas Al-Zyoud 3, Asem Abo-Alosh 4, Manal Halaybeh 5
1Researcher at the NCARE, Ph.D. in Entomology, Baq'a, 19381 Jordan
2Researcher at the NCARE, M.Sc. in Plant Protection, Baq'a, 19381 Jordan
3Associate Prof. of Biological Control and IPM, Department of Plant Protection and IPM, Faculty of Agriculture, Mu’tah University, Karak, 61710, Jordan
4Researcher at the NCARE, M.Sc. in Entomology, Baq'a, 19381, Jordan
5Researcher assistant at the NCARE, Al-Mushagger Regional Center, Madaba, Jordan
Correspondence to: Firas Al-Zyoud , Associate Prof. of Biological Control and IPM, Department of Plant Protection and IPM, Faculty of Agriculture, Mu’tah University, Karak, 61710, Jordan.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
The hibiscus mealybug, Maconellicoccus hirsutus Green (Hom., Pseudococcidae) is distributed throughout the world, and attacks a wide spectrum of host plants including Guava. M. hirsutus causes direct and indirect damages to the Guava plants. However, no attention has been paid on the effect of abiotic and biotic factors on M. hirsutus in Jordan. Therefore, the present study aimed at investigating the population dynamics of M. hirsutus on guava taken into account the effect of direction, time, temperature and associated parasitoid throughout the 2009/2010 growing season in Madaba District - Jordan. The results indicated that M. hirsutus nymphs have three peaks. The adult females appeared in early February with very low numbers, while the most presence of the females occurred in mid July. Males appeared also in early January and then in late July and early August. The highest number of the parasitoid, Anagyrus sp. was reported during February. According to the direction, adults were significantly the highest in North followed by West and South. In regards to all stages (eggs, nymphs and adults), the pest was significantly found more in East (391 individuals), followed by West (359 individuals) and South (350 individuals). In the other hand, the parasitoid individuals were significantly higher in North and West, followed by South. There was a positive and significant correlation between the number of M. hirsutus nymphs and the parasitoid, Anagyrus sp. (r = 0.444, P = 0.039), and M. hirsutus adults and Anagyrus sp. (r = 0.403, P = 0.050). Moderate mean temperature and RH seem to be favorable for the pest, since most of nymphs and adults occurred during these conditions. It is to be mentioned that the infestation by the pest reached up to 98%. In conclusions, the current study provides basic information about the population dynamic of the pest and its associated parasitoid, and this will help positively in controlling the pest in Jordan successfully.
Keywords: Maconellicoccus hirsutus, Hibiscus Mealybug, Parasitoid, Population Dynamics, Guava, Jordan
Cite this paper: Muna Al-Fwaeer , Ibtihal Abu-Obaid , Firas Al-Zyoud , Asem Abo-Alosh , Manal Halaybeh , Population Dynamics of the Hibiscus Mealybug Maconellicoccus hirsutus Green (Hom., Pseudococcidae) and Its Parasitoid on Guava Trees in Madaba-Jordan, International Journal of Agriculture and Forestry, Vol. 4 No. 3, 2014, pp. 171-177. doi: 10.5923/j.ijaf.20140403.04.
Article Outline
1. Introduction
- Guava, Psidium guajava L., originated from tropical America, is widely grown in tropical and subtropical regions of the world [1, 2]. In Jordan, guava is commercially grown in Jordan Valley and Highlands, i.e. Irbid, Madaba and Taphella Districts. The total planted area with guava trees in Jordan Valley and Highlands is estimated at 2,195 ha producing 16,350 tons [3]. It is expected that the commercial and backyard planting of guava trees in the country will continue to increase in the coming years.The hibiscus mealybug or pink mealybug, Maconellicoccus hirsutus Green (Hom., Pseudococcidae) has been inexorably spreading through the Caribbean Islands and it is now reported in eighteen different Caribbean Islands. The pest attacks a wide variety of host plants, and it has been rapidly and destructively spreading throughout many countries [4, 5]. M. hirsutus is a polyphagous pest attacking a wide range of crops, ornamentals, timbers and wild plants. It is found in tropical Africa, South East Asia, Northern Australia, Middle East, India and Pakistan [6]. However, the pest is firstly reported in Jordan in Ayoon Al-Deeb/Madaba District in 2009 [7].The hibiscus mealybug is become one of the most serious insect pests on guava in Jordan, causing damage to plant stems, leaves, buds and fruits by injecting a toxic saliva that causes curling and contortion to the leaves. Thus, the entire plant may stunt and the shoot tips develop bushy appearance. Furthermore, buds may not flower, stems twist, and fruits may also deform. Furthermore, M. hirsutus excretes honeydew which encourages the development of black sooty molds [8]. It is to be mentioned that in case of high M. hirsutus populations, the plant might be killed [2]. The overall annual costs of control and damage losses resulted from M. hirsutus to the United States of America economy have been estimated at 700 million dollars with a global total being about 5 billion dollars [9].Nevertheless, it was pointed out that abiotic and biotic factors affect the development, activity and reproduction of insect pests under which guava is growing. In addition, arthropod pests, natural enemies and plants are major biotic components of the agro-ecosystem. Accordingly, changes in weather conditions can be either helpful or harmful to pests' populations [10, 11, 12]. Understanding of such factors may contribute in designing a successful integrated pest management (IPM) program to control insect pest [13, 14]. However, up to date and to the best of our knowledge, no research has been undertaken on the effect of abiotic and biotic factors on M. hirsutus on guava in Jordan. Therefore, the present study aimed at investigating the population dynamics of M. hirsutus on guava taken into account the effect of direction, time, environmental conditions and associated parasitoid throughout the 2009/2010 growing season. It is hoped that this work could help positively in laying the foundation for sustainable and environmentally friendly pest control strategy through IPM for guava production in the future.
2. Materials and Methods
2.1. Experimental Site and Conditions
- The current study was carried out in Ayoon Al-Deeb/Madaba District of Jordan. The population dynamics of M. hirsutus and its associated parasitoid during the 2009/2010 growing season were thoroughly investigated on guava trees of Egyptian cultivar. The planting distance was 6x6 m (length and width) for the trees. Age of the guava trees averaged 15±2 years. Pesticides were not used throughout the entire period of the study. The trees were irrigated at 4-day-interval in summer and 12-day-interval in winter, pruned during January and organic fertilizers were added during the growing season. Records of temperature and relative humidity (RH) were obtained from Madaba Metrological Station at 10 km of the experimental site.
2.2. Population Dynamics of the Pest and Its Associated Parasitoid
- For studying the population fluctuation of M. hirsutus and its associated parasitoid, ten guava trees were randomly chosen and marked with a color sheet placed around the trees' trunk. Bi-weekly samples consisting of four 20-cm-terminal branch/tree were selected from each tree. Each branch was randomly collected from each tree's direction (North, South, East and West) starting from early January until late December in the growing season [1]. Sampling was taken place in the early morning, and the collected branches from each direction and tree were separately kept in polyethylene bags and transferred to the laboratory. The egg sacs, the pest immature stages (eggs, nymphs (N1, N2 and N3), adults' females and males of M. hirsutus as well as its associated parasitoid, Anagyrus sp. found on the branches were carefully examined by a Binocular Microscope, and the numbers of individuals were recorded. The relationship between the population density of the pest and the biotic factors (parasitoid) and abiotic factors (temperatures and RH) were reported over the period of the growing season. Furthermore, the pest infestation percentage was calculated using 100 guava trees.
2.3. Statistical Analysis
- In order to affirm the basic assumptions of the data to be analyzed, they were firstly tested for the normal distribution and homogeneity of variance using the Barlett-test [15]. When the data fulfilled the assumptions mentioned above, one-factor-analysis of variance was conducted to detect differences among means [16]. In case of differences among means were detected, then the second step was to determine the significant differences among means at a probability level of ≤ 0.05 using Least Significant Differences (LSD) Test [17, 18]. The statistical analysis was performed using the proc GLM of the Statistical Package SigmaStat version 16.0 [19]. In addition, the correlation between the population of the pest and both of weather factors and parasitoid was calculated by Spearman’s correlation method [20].
3. Results
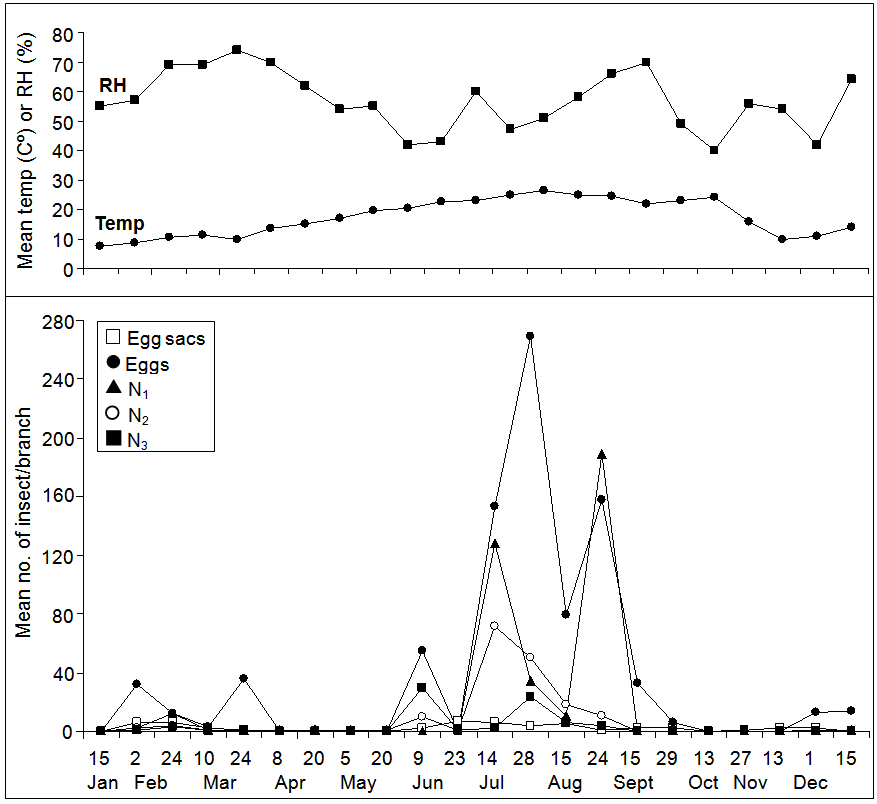
- The mean numbers of the hibiscus mealybug, M. hirsutus and its associated parasitoid on guava trees in 2009/2010 growing season are shown in Figure 1 and 2. M. hirsutus egg sacs were found in small numbers in January and reached a peak of 6 sacs/branch in early February, then the numbers declined throughout March to early June. The second peak was reached (7 sacs/branch) at late June. Thereafter, the sacs found in small numbers and fluctuated until no egg sacs were found after mid December (Figure 1). Eggs showed three small and two large peaks; the first three ones were occurred during early February, late March and early June, respectively, with a range of 32-55 eggs/branch. The fourth peak (269 eggs/branch), the largest one, was appeared in late July, followed by the fifth peak which appeared in late August with 158 eggs/branch. Then, numbers fluctuated until no eggs were found after mid December (Figure 1). The first nymphal instar appeared in small numbers (12 nymphs/branch) in February, and continued to fluctuate until late June. Then, the numbers increased dramatically and reached a peak of 128 nymphs/branch at mid July. A second peak was reported in late August with 189 nymphs/branch. The second nymphal instar showed a peak in mid July with 72 nymphs/branch, and then the number decreased and fluctuated until the end of the study. Two peaks of the third nymphal instars were reported; the first one was taken place in early June and the second one in late July with 30 and 24 nymphs/branch, respectively (Figure 1). Overall, for all nymphal instars together, three peaks were recorded. A small one was recorded in late February (19 nymphs/branch), and two large ones were occurred in mid July (202 nymphs/branch) and late August (204 nymphs/branch), respectively. The adult females appeared in early February with very low numbers, while the most presence of the females was occurred in mid July with 10 females/branch (Figure 2). Hereafter, the numbers decreased again until no females were found in late August. Males also started to appear in early January, and then in late July and early August until no males were recorded after late August. The highest number of the parasitoid, Anagyrus sp. was found in early and late February with 16 and 12 parasitoids/branch, respectively. The numbers decreased sharply and then fluctuated until no individuals were found after mid September (Figure 2).
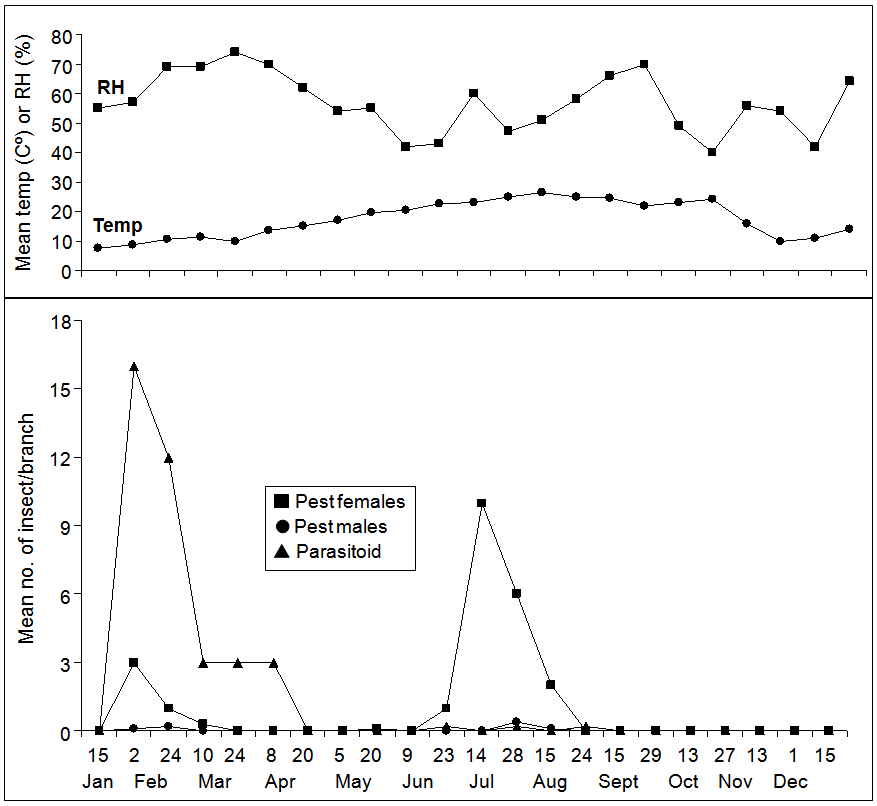 | Figure 2. Mean numbers of Maconellicoccus hirsutus adults' females and males as well as its parasitoid on guava trees in a field experiment in Madaba-Jordan in 2009/2010 growing season |
4. Discussion
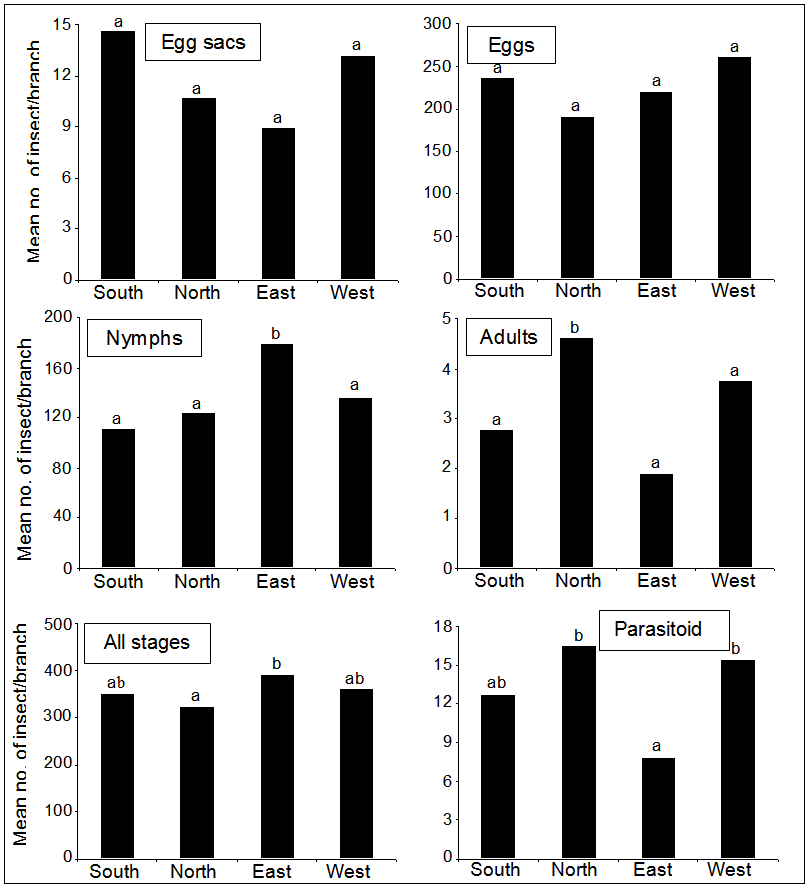
- The hibiscus mealybug is distributed in much of the world, and attacks a wide variety of host plants [5]. The pest causes direct and indirect damages to the plants [8]. Investigating the effect of abiotic and biotic factors on the development, activity and reproduction of M. hirsutus may positively contribute to suppress the pest [14]. However, the current results indicated that eggs show five peaks, in which the smallest one was in February and the largest one in July. This might be due to climate conditions, especially temperature, which served during that period and considered suitable for females to lay eggs. For nymphal instars, the three obtained peaks were in February, July and August. The small peak was appeared in February when the mean temperature was about 11°C, while in July and August the insect had the largest peak where the mean temperature was 24°C. However, previous studies indicated that seasonal climatic patterns influence the hibiscus mealybug populations. The pest populations were low during the winter season, and peaked during the summer months in South India [21], Caribbean [22], and Australia [23], which are in agreement with the current results. The present results showed that adult females appeared in February with very low numbers due to low temperature (about 13°C) and moderate RH (57%), while the most presence of adult females were reported in July when mean temperature and RH were 32°C and 53%, respectively. This result is in partial agreement with results of Chong et al. [24], who reported that the development of M. hirsutus is the fastest at 27°C. In addition, minimum and maximum as well as optimal temperatures' threshold for the development of mealybug females were estimated at 15-35°C and 29°C, respectively. Mani and Thontadarya [21] demonstrated that maximum temperature has a positive correlation, while RH has a negative correlation with the mealybug populations. The present research showed that the parasitoid, Anagyrus sp. showed the highest numbers in February. In this regard, fecundity was significantly affected by temperature and photoperiod [25]. The low number of the parasitoid that emerged from parasitized pest might refer to the application of pesticides in the surrounding areas of the research location, which might affect the natural enemies, or due to hibiscus mealybugs (PHMs) defense mechanism. Similar results were obtained by other researchers, in which they reported that 60% of the parasitoid eggs lays in adult female PHM did not develop due to PHM,s cellular defense of encapsulation and melanization of the parasitoid eggs [5, 26, 27]. In addition, there are some other factors that could reduce the effectiveness of biological control agents including the using of insecticides and sugar-loving ants that will protect PHM from parasitoids and predators [28]. In regards of direction, the pest adults were more reported in North and West, while egg sacs and eggs were presented in South and West following the adult even no significant differences were detected among the four directions. The adults present in February with high numbers in North and East. This might be due to that adults search for a protected location from low temperature. This is in agreement with results obtained by Mani and Thontadarya [21], who reported that PHM can adapt to cooler weather by nymphs moving to sheltered locations, and adult females choose protected places for laying egg sacs. Results of this study showed that the distribution range of M. hirsutus expands in the North and East directions because of the lower minimum temperature, which is going alone with conclusions made by Chong et al. [24].Furthermore, the results indicated that the pest nymphs present in East and West. The USDA response guidelines suggest that eggs, crawlers and adult males have the potential to be blown by wind currents in the upper atmosphere [29]. Mealybugs have also been shown to disperse aerially and via walking. Furthermore, the distribution and movement of the mealybugs on orange trees were observed to be in different numbers according to the various parts of the plant throughout the year, while with similar patterns each year [30]. Females would not disperse if they are already in a protected site, but would reproduce there [30]. Also, Goolsby et al. [23] reported that the pest populations were low during the rainy winter season, and peaked during the summer months. In cooler climates, females remained inactive in sheltered parts of the plant or overwintered as egg stage. Our study showed a positive significant correlation between the number of both pest's nymphs and adults with the parasitoid, and this was obvious from the presence of the parasitoid and pest's adult in the same direction (i.e. North and West). This is logic because the parasitoid possesses positive density dependence on prey. This agrees with the results of Sagarra et al. [25]. In conclusions, the current study provides basic information on the population dynamic of the pest and its associated parasitoid, and this will help in controlling the pest successfully in Jordan. Therefore, it is recommended that much attention should be directed to this pest in the future. Furthermore, it is hoped that this work could help positively in laying the foundation for sustainable and environmentally friendly pest control strategy through IPM for the pest on guava in the future.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML