-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2014; 4(2): 112-117
doi:10.5923/j.ijaf.20140402.11
Control of Cucumber Damping-off in the Field by the Bio-Agent Trichoderma harzianum
Nofal. S. Al-Ameiri
Assoc. Prof. of Plant Pathology, Dept. of Plant Protection and IPM, Faculty of Agriculture, Mutah University, Karak, 61710, Jordan
Correspondence to: Nofal. S. Al-Ameiri, Assoc. Prof. of Plant Pathology, Dept. of Plant Protection and IPM, Faculty of Agriculture, Mutah University, Karak, 61710, Jordan.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
Biological control of plant pathogens is becoming an important component in plant disease management. Pythium aphanidermatam is one of the most serious pathogens in cucumber at seedling stage, and can cause both pre- and post-emergence damping-off. Trichoderma spp. appeared to be effective in controlling soil-borne pathogenic fungi. Therefore, the present study aimed at assessing the efficacy of Trichoderma harzianum in controlling cucumber damping-off under field conditions. The current field study was carried out at Agricultural Research Station, Faculty of Agriculture, Mutah University, Jordan during the growing season (April-May, 2012). Six different treatments were used in this study. Disease incidence and severity (pre- and post-emergence damping-off), and disease control percent were recorded. Results indicated that adding T. harzianum to the infested soil significantly reduced pre-emergence damping-off in cucumber. On contrast, in case of post-emergence there were no significant differences between the pathogen and the treatments using the bio-agent as conidia or conidial preparation. In the mycelium and mycelia preparation treatments, the damping-off decreased significantly when compared to all treatments. Addition of all bio-controling agents reduced significantly total damping-off. The highest reduction in damping-off percentage was recorded by T. harzianum when used as a mycelial preparation where it reached 70%. T. harzianum decreased disease severity to 16% in mycelial preparation treatment when compared to P. aphanidermatum treatment, where it reached up to 73%. In conclusion, T. harzianum has the ability to suppress damping-off in cucumber caused by P. aphanidermatum under field conditions, and increases the efficiency by supply foods to stimulate development by increasing the population and increase antibiotic products.
Keywords: Trichoderma harzianum, Pythium aphanidermatam, Damping-off, Biological Control, Cucumber, Jordan
Cite this paper: Nofal. S. Al-Ameiri, Control of Cucumber Damping-off in the Field by the Bio-Agent Trichoderma harzianum, International Journal of Agriculture and Forestry, Vol. 4 No. 2, 2014, pp. 112-117. doi: 10.5923/j.ijaf.20140402.11.
1. Introduction
- Biological control of plant pathogens is becoming an important approach in plant disease management (Cook and Baker, 1983). The general mechanism of biological control can be divided into direct and indirect effects against plant pathogens. Direct effects of the bio-agent include: competition for nutrients or space against pathogens, production of antibiotic and lytic enzymes, inactivation of the pathogen’s enzymes and parasitism (Paulitz et al., 1990; Al-Ameiri, 2001, 2009; Mohiddin et al., 2010; Khan et al., 2011 El-Hasan et al., 2013). Indirect effects include all those aspects that produce morphological and biochemical changes in the host plant such as: increase tolerance to stress through enhancing root and plant development, solubilization or sequestration of inorganic nutrients, and stimulate plant self resistance against diseases (Viterbo et al., 2002; El-Hasan et al., 2013).The soil Trichoderma spp. suppresses damping-off caused by soil born agents and active colonizers in soil (Hadar et al., 1979; Elad, et al., 1980; Akramin et al., 2009). Various reports in the literature demonstrate that Trichoderma spp. is already used in both laboratory or greenhouse experiments to control fungi, nematodes and bacterial diseases (Lewis and Papavizas, 1985; Mohamed and Abu-Raya, 1993; Adm, 2000, Al-Ameiri, 2001, 2009). Different isolates of Trichoderma spp. appeared to be effective in controlling any array of soil-borne pathogenic fungi (Hadar et al., 1979; Lewis and Papavizas, 1985; Mohamed and Abu-Raya, 1993; Al-Ameiri, 2001, 2009). Trichoderma spp. has been also used to protect seeds of cucumber, tomato, okra and pea against Pythium damping-off under both greenhouses and field conditions (Hadar et al., 1984; Harmen and Tyler, 1988; Paulitz et al., 1990; Adm, 2000, Al-Ameiri, 2001). Trichoderma harzianum antagonizes Pythium aphanidermatam by parasitism and antibiosis (Paulitz et al., 1990; Al-Ameiri, 2001, 2009). Naraghi et al (2014) found that the Antagonistic effects of Talaromyces flavus and Trichoderma harzianum on sugar beet damping-off in the greenhouse and field were superior to carboxin-thiram and control in terms of number of healthy seedlings. However, Meller Harel et al. (2014) that they found the plants that treated with 0.04% Trichoderma harzianum T39 drench exhibited 62% less severe disease than the untreated plants and 84% reduction in disease severity.The genus of Pythium includes a number of readily recognized species with a wide distribution and host range (McCarter and Littrell, 1970). Pythium aphanidermatam is one of the most serious pathogens of cucumber, tomato and sugar beet at seedling stage causing both pre-and post-emergence damping-off (McCarter and Littrell, 1970; Patel and Patel, 1975; Adm, 2000; Al-Ameiri, 2001). P. aphanidermatum favor temperature range from 27°C to 34°C, wet conditions with preference to slight acidic media (pH = 6.2) (Lumsden et al., 1976). Furthermore, the numbers of propagules are increased through August and September while decreased in March and April (Leach, 1947; Dewan, 1994).The present study aimed at assessing the efficacy of T. harzianum in controlling cucumber damping-off caused by P. aphanidermatum under Jordanian field conditions.
2. Materials and Methods
- The current study was carried out under field conditions at the Agricultural Research Station, Faculty of Agriculture, Mu'tah University, Karak, Jordan (Latitude: 3116 N; Longitude: 3545 E; Elevation: 920 m) during growing season (month-month, 2012). The soil had the following characteristics: pH of 7.2, total N of 0.22%, P of 160 ppm, K of 350 ppm and 2.1% organic matter.Inoculums' isolate of P. aphanidermatum was isolated from infected cucumber plants. Dead infected seedling at the stem base and rotting roots in post-emergence damping-off of cucumber seedlings were rinsed in tap water for an hour, disinfected by 0.50% sodium hypochlorite, then rinsed in a sterile water, dried on filter paper and then plated on potato dextrose agar (PDA). The samples were identified according to Waterhouse (1968), maintained on PDA, and the inoculates were incubated at 22°C in 10x3x2 cm glass tubes covered with aluminum foil. T. harzianum was isolated from the same soil by dilution method plating and identifying according to Rifai (1969), and mutagenesis by benomyl tolerant as described by Ahmad and Baker (1987), and they were grown on PDA containing 10 µg a.i. benomyl/ml.The experimental area was covered by plastic mulch and irrigated by a mixture of fungicides; benomyl-50 (50g/m) and tachigazol (50ml/m) at one-week-intervals to control any soil pathogens. One month later the mulch was removed and the area was divided into three blocks with 2 m distance between the blocks, in which each block was contained 6 treatments, each of 3 m long and 0.75 m between lines. The soil from each line was removed at 5 cm depth, 15 cm width, and 300 cm length to add and mix the inocula of the experimental treatments, then covered with soil.Six treatments, each with three replicates (lines) of 3 m long, were arranged in the field in a completely randomized block design (CRBD). The treatments were: (1) Control, no pathogen or bio-agent: 25 g of autoclaved wheat seeds/m of soil lines to assess seed germination of cucumber. (2) P. aphanidermatum (P. aph.) alone: 25 g of infected wheat seeds/m of soil line (2500g of wheat seeds and 25000ml of water (at 10 flasks of one liter) were autoclaved at 121°C for 1 h twice, then cooled, and 100ml of conidia suspension of P. aphanidermatum were directly added to autoclaved wheat seeds. The flasks were incubated for 2 weeks, and then dried at room temperature, and 25g/m of soil lines were used in the pathogen's treatments. (3): Conidia suspension (Con. Sus.) of T. harzianum grown on PDA at 25°C was harvested by scraping the surface of the colonies with a spatula and transferred to flask of water to give spores concentration of 107/ml and 100 ml/m of soil lines added. (4): Conidial preparation (Con. Pre.) 2500g of wheat bran and 2500ml of water (at 10 flasks of one liter) were autoclaved at 121°C for 1 h twice, and then cooled. 100ml of the above mentioned conidial suspension were directly added to wheat bran. In addition, 25g/m of soil line were added as a conidial preparation before incubation. (5): Mycelium (Myc.) in which potato dextrose (PD) medium was autoclaved, and hereafter cooled, seeded with 0.5 cm of T. harzianum disc and incubated for three days. The mycelium was filtered by using filter paper (Whitman number ), and the filtration was repeated three times, and washed to be free from nutrients before adding it to the soil (15g/m of soil line). (6): Mycelial preparation (Myc. Pre.) where a modification of the wheat bran culture (seed as a carrier and food substance for T. harzianum to increase effectiveness) for the bio-agent was used (Hadar et al., 1979). The wheat bran was autoclaved, and then inoculated by the above mentioned concentration of conidial suspension, and hereafter was incubated for five days and then dried at room temperature for three days. It was used in average by 25g/m of soil line.The soil was inoculated with bio-agent and the pathogen in the treatments 3, 4, 5 and -6. After three days from inculation, fifteen cucumber (var. Beit Alpha) surface-sterilized seeds were sown in each soil line, and watered daily by drip irrigation. Disease incidence (pre- and post-emergence damping-off) was calculated ten days later. The number of seeds, which did not germinate (pre-emergence) and pre-emergence damping-off ((pre-emergence/total number of seeds/line) x 100%) were also calculated. The percentage of damping-off of post-emergence after three weeks (number of dead seedlings and infected seedlings ((post-emergence)/total number of seeds/line) x 100%) was recorded. Damping-off seedlings were routinely grown on PDA to identify the pathogen. Disease severity and percent of disease control were recorded. Disease severity was calculated on a scale of 0-3 according to Van Dijk and Nelson (1998) at the following scheme:
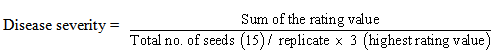 Where 0: healthy seedlings, 1: diseased seedlings (infected seedlings and did not die during the experimental period and lesions appeared brown in color at lower stem near the soil line), 2: dead post-emergence and 3: dead pre-emergence. The percent of disease control was calculated using the formula according to Abbot (1925):
Where 0: healthy seedlings, 1: diseased seedlings (infected seedlings and did not die during the experimental period and lesions appeared brown in color at lower stem near the soil line), 2: dead post-emergence and 3: dead pre-emergence. The percent of disease control was calculated using the formula according to Abbot (1925):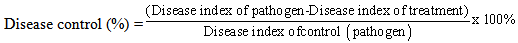 The statistical analysis was performed using the proc GLM of the statistical package Sigma Stat version 16.0 (SPSS, 1997). The data were analyzed using one way ANOVA to detect any differences in the studied parameters (Wilkinson, 1990; Zar, 1999). When significant differences were detected, means were separated using LSD at 0.05 probability level (Abacus Concepts, 1991). The control treatment was not included in the statistical analysis for disease incidence and severity parameters.
The statistical analysis was performed using the proc GLM of the statistical package Sigma Stat version 16.0 (SPSS, 1997). The data were analyzed using one way ANOVA to detect any differences in the studied parameters (Wilkinson, 1990; Zar, 1999). When significant differences were detected, means were separated using LSD at 0.05 probability level (Abacus Concepts, 1991). The control treatment was not included in the statistical analysis for disease incidence and severity parameters.3. Results
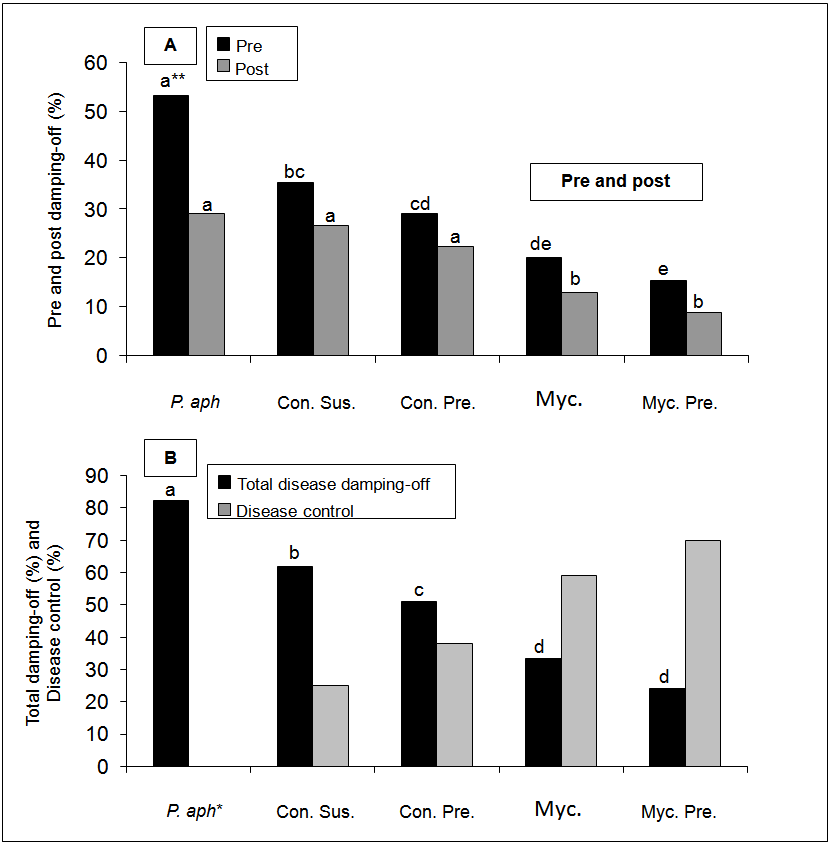
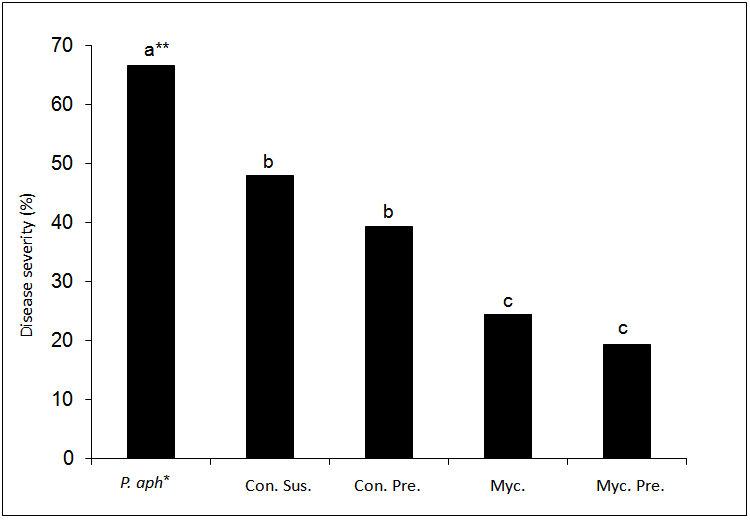
- The addition of T. harzianum to the infested soil reduced significantly pre-emergence damping-off of cucumber when compared to P. aphanidermatum alone (Figure 1A). Pre-emergence damping-off with P. aphanidermatum alone reached to 53.3%, while with adding the bio-agent it reached 35.3% with spores' suspension treatment and 15.3% with mycelia preparation treatment, thus, this treatment showed the greatest reduction in damping-off caused by P. aphanidermatum. In case of post-emergence, there were no significant differences between the pathogen and the bio-agent treatments such as conidia or conidial preparation, but there was a numerical decrease in damping-off in these treatments. In the mycelium and mycelia preparation treatments, the damping-off decreased significantly when compared to other treatments (Figure 1A). The post-emergence damping-off decreased from 29.3% with the pathogen to 9.8% with the bio-agent mycelia preparation treatment.The addition of all bio-agent treatments decreased significantly the total damping-off (Figure 1B). Total damping-off reached up to 82.2% in the pathogen treatment, while disease percentage decreased to 24.6% with the bio-agent treatment (mycelia preparation). The efficiency of T. harzianum in controlling disease incidence is presented in Figure 1B. The results revealed that all tested bio-agents were suppressed damping-off caused by P. aphanidermatum. Concerning the method of application, these results indicated a variation among treatments. The highest percent of damping-off disease reduction was recorded by T. harzianum when used as a mycelial preparation (70%), followed by mycelium treatment (59%), and was the lowest in conidial suspension treatment (25%).
4. Discussion
- The current study was conducted in order to investigate the capability of T. harzianum to reduce cucumber damping-off disease caused by P. aphanidermatum under field conditions. Different methods were used to apply T. harzianum to reduce disease incidence and severity under greenhouse conditions. However, this study is a unique since it is the first study to investigate the efficiency of T. harzianum under Jordanian field conditions to control P. aphanidermatum that caused severe cucumber damping-off.The results of the current study indicated that under field conditions all application methods of the bio-agent, T. harzianum caused a significant reduction in the pre-emergence damping-off disease. In case of post-emergence treatments, there were no significant differences between the pathogen and treatments using bio-agents as conidia or conidial preparation. These results are in agreement with those of Hadar et al. (1979), Harmen and Taylor (1980), and Al-Ameiri (2001). T. harzianum applied to the soil as a mycelium and mycelial preparation decreased pre- and post-emergence damping-off under greenhouse conditions. This might be due to the fact that it contains young active growing hyphae and abundance of chlamydospores, and the pathogens were directly become observed with the active structure of the mycelium bio-agent. These results may indicate that the conidia that added to the soil under field conditions were ineffective in decreasing the disease. Ineffectiveness of conidia to suppress P. Aphanidermatum infestation can be attributed to two possible reasons. Firstly, no enough time was available to produce hyphae that are able to parasite upon and destroy the pathogen .Secondly; conidia may be exposed to leaching as a result of irrigation through the soil pores as compared to mycelium. These results are in agreement with the results of Lewis and Papavizas (1984) and Al-Ameiri (2001), in which they found that the conidia were less effective against P. Aphanidermatum than mycelium when applied to soil. Amsellem et al. (1999) reported that mycelial and chlamydospores are more tolerant to environmental conditions during product formulation and field use than conidia. However, the presence of the mycelia mass is also a key component for the production of antagonistic metabolites (Benhamou and Chet, 1993; Yedidia et al., 2000).Current study also indicated that all methods of bio-agent' application gave a significant reduction of the total damping-off disease as compared with the pathogen treatment. Similar results were also reported elsewhere (Adam, 2000; Al-Ameiri, 2001; Howell, 2003; Meller Harel et al. (2014). The percentage of disease control was ranged from 25% to 70% when applying the bio-agent. Our study showed that T. harzianum application was effective in reducing disease severity significantly as compared to P. aphanidermatum treatment. This difference might be attributed to the efficiency of application method and the ability of pathogen to utilize from the wheat seed that used as a carrier and food substance which gives a high stimulation for the development of P. aphanidermatum and the ability of Pythium spp. to live as saprophyte fungi and increase infection of hosts (Holmes et al., 1998; Al-Amieri, 2001; Meller Harel et al. (2014).In conclusion, our results demonstrate that the bio-agent, T. harzianum has the ability to suppress the damping-off of P. aphanidermatum under field conditions, and increase the efficiency by supply foods to stimulate the development by increasing the population and antibiotic products.
ACKNOWLEDGMENTS
- Author would like thank Dr. Firas Al-Zyoud for his assistant in statistical analysis.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML