-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2014; 4(2): 67-72
doi:10.5923/j.ijaf.20140402.03
The Effect of Salinity and Glycine-Betaine Application on Some Plant Hormones Production by Different Rice Varieties
Fusun Yurekli
Inonu University, Science and Arts Faculty, Department of Biology, Campus 44280 Malatya Turkey
Correspondence to: Fusun Yurekli, Inonu University, Science and Arts Faculty, Department of Biology, Campus 44280 Malatya Turkey.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Plants have mechanisms to cope with undesirable conditions such as water-deficient or saline conditions. Plant hormones, enzymes and various solutes make plant to tolerate abiotic stress. Glycinebetaine is well known among these solutes. Some plants can not synthesize and accumulate glycinebetaine in stressful conditions. We have investigated to evaulate the effects of glycine betaine and glycinebetaine plus sodium chloride on plant hormones levels in the rice varieties Pokkali and IR-28. Exogenous application of glycinebetaine resulted in an increase in indole acetic acid (IAA) and abscisic acid (ABA) and a decrease in zeatin level. Our comparative study suggests that exogenous glycinebetaine application lead to changes hormone level after salinity by increases or decrease and accompany increasing in ABA that protects plants against the salt stress. Due to the application of glycine betaine and salinity ABA and IAA levels increased in tolerant Pokkali and sensitive IR-28 varieties. Zeatin levels increased in Pokkali and reduced in IR-28 varieties.
Keywords: Salinity, Glycinebetaine, Stress, Plant Hormones, Rice
Cite this paper: Fusun Yurekli, The Effect of Salinity and Glycine-Betaine Application on Some Plant Hormones Production by Different Rice Varieties, International Journal of Agriculture and Forestry, Vol. 4 No. 2, 2014, pp. 67-72. doi: 10.5923/j.ijaf.20140402.03.
1. Introduction
- Abiotic stresses are prevalent in nature and can substantially diminish plant yields. Plant responses to stressful environmental factors can be one of the mechanisms that permit the plant to withstand the stress. Alternatively, such responses may be a manifestation of injury that has occurred in response to the stress. Most organisms accumulate highly soluble compounds termed osmoprotectants or osmolytes under stresses such as salinity, drought and cold. One of the most-important osmoprotectants is glycine betaine, GlyBet, which is widespread and accumulates in organisms from archaebacteria to higher plants and animals [1]. Previous reports show that betaine in vitro can protect enzyme activity and stabilize the cell membrane ([2-6]. Betaine in vivo can not only have these effects but also regulate the osmotic pressure in the cell, by reducing the damage to the cell from by abiotic stresses [7,8]. Some flowering plants, e.g. sugar beet, accumulate high levels of GlyBet [9], whereas in many crops, such as rice and wheat, osmoprotectant content is very low. It is thus possible to improve the stress tolerance of these crops by introducing genes responsible for GlyBet biosynthesis. Advances have been made in recent years in plant metabolic engineering of trehalose [10], and GlyBet [11,12]. The plant’s response depends on the severity and duration of the stress, the developmental stage of the affected plant, the tissue type, and the interactions of multiple stresses. Mechanisms that permit stress survival are termed resistance mechanisms and can allow an organism to avoid or tolerate stress. Stresses involving water deficit may arise from drought conditions, saline soils, or low temperature. Measuring the water status of the plant is important for determining the impact of the environmental condition. Decreases in plant water potential may be brought about by osmotic adjustment, the accumulation of compatible solutes that promote acclimation to dry or saline soils. Compatible solutes, such as glycine betaine, mannitol, pinitol, and proline, do not disrupt cellular function when they reached high concentrations in the cytoplasm. In addition to osmotic adjustment, some compatible solutes may serve other protective functions. Stress-related signals are propagated by several different agents. In a saline area, salt ions attract water, raising the suction of water head in the soil, thereby reducing the water potential of plant tissues. The low water potential directly affects the efficiency of water use, because plants need to develop more negative water potential to maintain a downhill gradient of water potential between the soil and the plant [13]. Moreover, salts in saline soil directly damage the plant cells, tissues and organelles. Salt-sensitive species are particularly susceptible to injury by salt stress, resulting in the loss of leaf expansion and chlorophyll synthesis prior to plant death. In addition, many salt-stressed plant species show symptoms of damage symptoms such as wilting, chlorosis, necrosis, burn and senescence, resulting in low growth and productivity [14,15]. When plants are challenged with hyperosmolarity, the accumulation of ions such as Na+ in the vacuoles can lower the osmotic potential of the cells, and this process may be cost-effective with regard to the amount of energy and resources spent [16]. A related strategy used to lower the osmotic potential of the cell cytosol is to accumulate compatible osmolytes. For glycophytes, the capacities for Na+ compartmentalization and accumulation of osmolytes are both limited. Various compatible osmolytes such as proline, glycine betaine, and polyols can greatly reduce stress damage to plant cells. Increased production of osmolytes is a general phenomenon found in all plants in response to salt stress. This is clearly an adaptive strategy and transgenic plants with increased osmolyte production or reduced degradation [17] show improved salt tolerance. Besides reducing the osmotic potential of the cytosol to facilitate water uptake, many compatible osmolytes have additional functions such as protecting proteins from misfolding and alleviating the toxic effect of reactive oxygen species generated by salt stress [18,19]. The osmoregulation system is one of the defensive responses to abiotic stresses such as salt, drought, extreme temperature and high light intensity [1,20-23]. Osmoregulants, proteins, carbohydrates and amino acids play an important role in osmotic adjustment and stabilization of plant cells [24-26]. Glycinebetaine is a common compatible solute that accumulate in many species of Poaceae, Amaranthaceae, Asteraceae, Malvaceae, and Chenopodiaceae but is absent from many crop species such as carrot, soya bean, castor bean, tobacco and mustard [1,27]. The main role of Glybet is probably to protect plant cells against the ravages of salt stress by preserving the osmotic balance [28], stabilizing the structure of key proteins such as Rubisco [29], protecting photosynthetic apparatus such as reaction centre complexes [30] and functioning as oxygen radical scavengers [31]. The accumulation of Glybet could contribute significantly to chloroplast osmotic adjustment, facilitating maintenance of chloroplast volume and photosynthetic capacity at low leaf water potential [32, 33]. Thus, accumulation of glycine betaine has been linked with plants’ ability to better survive osmotic: water stress conditions. However, the impact of exogenous glycine betaine on plant water relations and on plants’ response to water stress is not clear. In this study, we determine the effects of exogenously applied glycine betaine on the ability of bean plants to withstand salinity as indicated by plant water relations and growth characteristics. In some cases, these signal transduction events involve some of the best-studied plant hormones: ABA, auxins, cytokinins, ethylene, and gibberellins. Increases in some growth factors concentrations in plant tissues correlate with growth promotion/inhibition in salt-stressed plants. Abscisic acid (ABA), a widely known signal for environmental stresses in plants [34,35], has been reported in recent years to possibly play a role in water-stress-induced betaine accumulation in plants [36-38]. Briefly auxins are need to promote initiation of adventitious roots and they can also be used to promote uniform flowering, to promote fruit set, and to prevent premature fruit drop; In general, abscisic acid it acts as an inhibitory chemical compound that affects bud growth, seed and bud dormancy. It plays a role in leaf and seed dormancy by inhibiting growth [39]. Cytokinins are a group of chemicals that influence cell division and shoot formation. They also help delay senescence or the aging of tissues. They have a highly synergistic effect in concert with auxins and the ratios of these two groups of plant hormones affect most major growth periods during a plant's lifetime. Cytokinins counter the apical dominance induced by auxins [40].This is the first investigation that has been performed on the concentration of ABA, IAA and zeatin following the exogen application of NaCl+Gly betaine. In the present study, the effect of glycine betaine on the production of ABA, IAA and zeatin was tested on the growing leaf that changes rapidly within 6 days, in response to salinity. Plants were exposed to NaCl and NaCl+Gly betaine and ABA, IAA and zeatin content was assass over.
2. Material and Methods
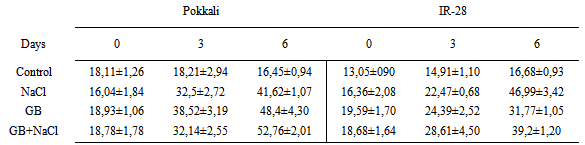
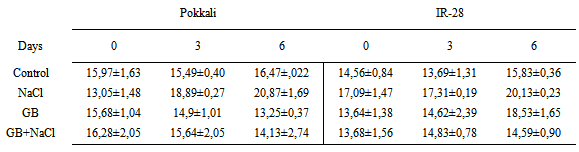
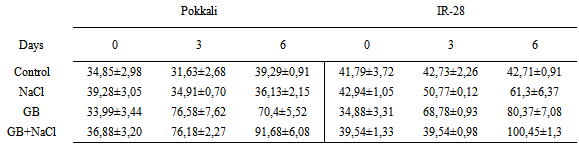
- Rice cultivars Pokkali (salt-tolerant) and IR-28 (salt-sensitive) were obtained from IRRI (International Rice Research Institute, Los Banos, Philippines). Plants were divided into four groups; Control (without GB and NaCl), GB, NaCl and GB+NaCl. Plants were grown for 4 weeks. Seedlings were provided with 15 mol m-3 glycinebetaine and 120 mol m-3 NaCl in growth chamber Seeds were sterilized with 0.1% SDS for 20 min and transferred to two sheets of sterile fitler paper after swelling seeds in deionized water at 28℃ for 6 h and then seeds were germinated at 28℃ for 72 h in plastic trays. Germinated seeds were grown in growth chamber at 27±2℃, 350 µmol m-2 s-1 light intensity and 60-70 % relative humidity. Nutrition solution was prepared according to Yoshida et al. [41]. ABA, IAA and zeatin were extracted and purified as described by Yurekli et al. [42]. At the end of the experiments, samples were injected into a C18 reverse-phase HPLC (Cecil 1100, Cambdridge, UK) fitted with a 4.6 i.d. 250 mm column. The column was eluted at a flow rate of 1.5 mL min-1 for ABA with 20–75% methanol in 0.4% acetic acid and at a flow rate of 1.5 mL min-1 for IAA and zeatin with 20-80% methanol in 10% acetonitrile by a linear gradient. The data obtained were analyzed using a SPSS for Windows 10.0 software package. Means and Standard errors were calculated for each group. The LSD was applied to estimate significant differences of metal levels in plant material using the ANOVA for p < 0.05.In the present study salt-tolerant Pokkali and salt-sensitive IR-28 were treated with NaCl, Glybet and NaCl+Glybet. NaCl exposure influenced the level of indole-3-acetic acid, zeatin and abscisic acid in Pokkali and IR-28. After glycine betaine and glycine betaine+NaCl treatment, the zeatin level decreased in Pokkali and slightly increased in IR-28. Glycine betaine treatment caused an increase in IAA and ABA level and GB+NaCl treatment caused additional increases in the plant hormones. In our study, IAA, zeatin and ABA levels were increased in Pokkali and IR-28 on days 3 and 6 after NaCl exposure. The highest levels of IAA were 52.67± 2.00 μg/mL and 39.20±1.20 μg/mL observed as 3.20-fold and 2.35-fold in Pokkali and IR-28 respectively after NaCl+GB treatment (Table 1). Zeatin levels were 14.13±2.74 μg/mL and 14.59±0.90 μg/mL in Pokkali and IR-28. These findings demonstrated that the treatment of plants with GB contributes to an increase in the IAA and ABA levels after salinity stress. The differences in the level of zeatin between GB and GB+NaCl were not found to be important (P>0.05). However, the treatment with GB and GB+NaCl slightly reduced/increased the zeatin level compared with control (Table 2). Salinity and salinity plus glycine betaine resulted in increased ABA levels in Pokkali and IR-28. In addition, the ABA level increased 2.1-fold and 2.03-fold in Pokkali and IR-28 respectively on day 6 after GB+NaCl treatment. The highest levels of ABA were 91.68±2.27 μg/mL and 100.45±1.30 μg/mL in Pokkali and IR-28 on day 6 (Table 3).
3. Results
|
|
|
4. Discussion
- Glycine betaine has been known to accumulate in a wide range of plants typically in response to salt and drought stress. It is established that plants can tolerate and adapt to sublethal levels of a variety of stresses, including the abiotic stresses caused by low and high temperatures drought, salinity and injury. The molecular mechanism of adaptation to abiotic stresses in plants is quite complex. The plants exposed to stress can modify their essential metabolic processes and are also able to synthesize enzymes, regulators and metabolites. Abscisic acid plays a primary regulatory role in the plant’s response to stress. The level of abscisic acid fluctuates dramatically in response to developmental and environmental changes. Abscisic acid stimulates the synthesis of many classes of proteins during stress. These proteins may protect membranes and other proteins from desiccation damage, or may aid in recovery from the deleterious effects of stress [43]. One of the most important roles of auxin in higher plants is the regulation of elongation growth in young stems and coleoptiles. Low levels of auxin are required for root elongation, although at higher concentrations auxin acts as a root growth inhibitor [44]. Cytokinins are generally required for cell division of plant cells in vitro. Cytokinins also play key roles in the regulation of cell division in vivo. Cytokinins participate in the regulation of many plant processes, including cell division, morphogenesis of shoots and roots, chloroplast maturation, cell enlargement, and senescence [45]. Salinity is one of the major limiting environmental factors in plant growth and development. Under salt stress, plants have to cope with water stress imposed by the low external water potential and with ion toxicity due to accumulation inside the plant. Salt stress induces the expression of specific genes and metabolic modifications, particularly the synthesis of osmoprotectans such as glycine betaine. Glycine betaine, one of the structurally simple betaines, was first extracted from the sugar beet and was found to have chlorophyll-retention properties. In growth tests it was found to have activity similar to that of cytokinins in several other growth tests and so it was proposed that some of the cytokinin-like activity in sugar beet extracts was due to glycine betaine. It has also been shown also to be a major osmoticum (controlling water movement in and out of plant cells) in certain higher plant families adapted to either salt or water stress. Biochemists have found that betaine-glycine is responsible for retaining chlorophyll in plant cells. A plant hormone that influences cell division, plant metabolism, and the synthesis of RNA, cytokinin properties discovered in sugar beets and marine seaweed extracts have been attributed, at least in part, to betaine-glycine. Through additional extract experiments, betaine-glycine was also found to be a major controller of water movements in and out of higher plant cells, particularly those species adapted for aquatic or salt-stressed habitats. It has been reported that glycine betaine level increased during cold treatment, suggesting that it is involved in the development of freezing tolerance in respond to cold treatment [37]. In addition, proline and glycine betaine have protective effects during stress treatment and also increase plant hormones. The increase of abscisic acid is especially remarkable [46,47]. It is conveciable that in response to stress, physiological and biochemical reactions act together after glycine betaine is exogenously applied and as a result of this, plant hormone levels can change. In response to salt conditions, closely related species cannot develop different tolerances. For example, there couldn’t have been a difference in leaf expansion ratio between salt sensitive durum wheat and salt tolerant barley after 10 days of salt treatment. With regard to salt exposure time, in salt treated plants difrerent mechanisms can be activated to control growth [48].It can be assumed that when plants are subjected to stress, biochemical and also hormonal responses can occur. It has been reported that depending on the salt stress applicitaion, plant hormone and glycine betaine levels increased, but there was no data about the increase of plant growth hormones (-IAA, zeatin, ABA). It was suggested that besides the protective effect of glycine betaine after its exogenous, plant growth hormone levels except zeatin increased. In order to understand this mechanism, additional studies should be conducted, especially on enzyme actions in plant hormone synthesis after internal/external glycine betaine application during stress conditions.
ACKNOWLEDGEMENTS
- The author would like to express her special thanks to Professor Ismail TURKAN of the Ege University, Science Faculty for his execellent advice of the manuscript.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML