-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2014; 4(1): 34-40
doi:10.5923/j.ijaf.20140401.05
Field Utilization of Nitrogen Fertilizers for Controlling 
 Root-knot Nematode and Improving Growth and Yield of Cucumber
Root-knot Nematode and Improving Growth and Yield of Cucumber
Muwaffaq R. Karajeh1, Farah M. Al-Nasir2
1Plant Protection and IPM Department, Faculty of Agriculture, Mutah University, Karak P.O. Box 7, 61710, Jordan
2Plant Production Dept., Faculty of Agriculture, Mutah University, Karak P.O. Box 7, 61710, Jordan
Correspondence to: Muwaffaq R. Karajeh, Plant Protection and IPM Department, Faculty of Agriculture, Mutah University, Karak P.O. Box 7, 61710, Jordan.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
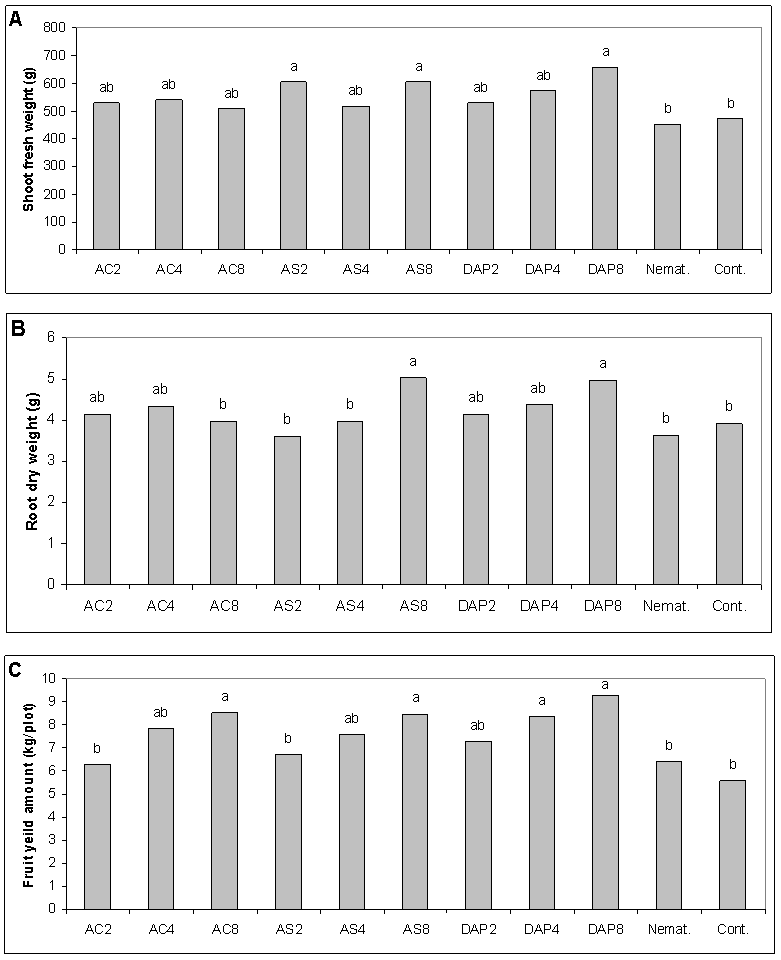
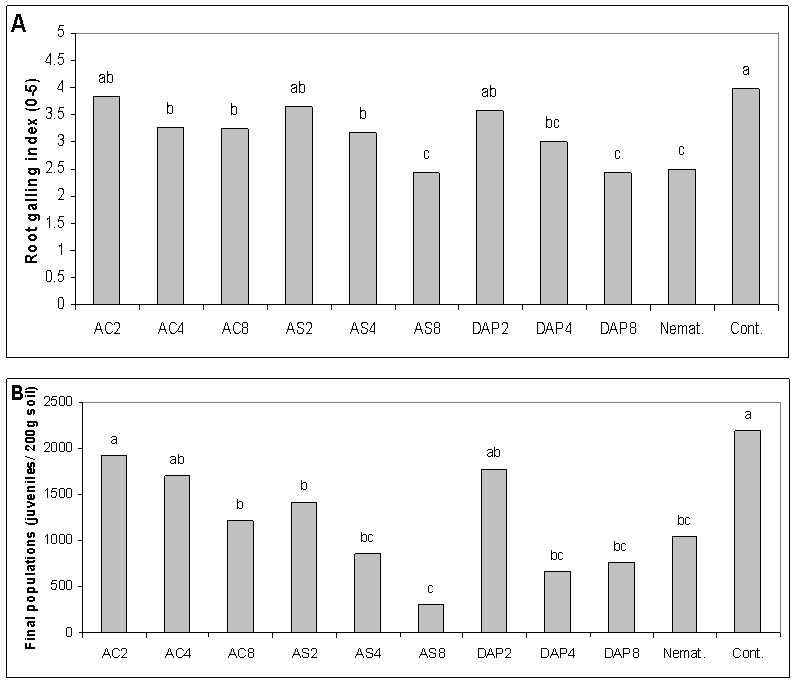
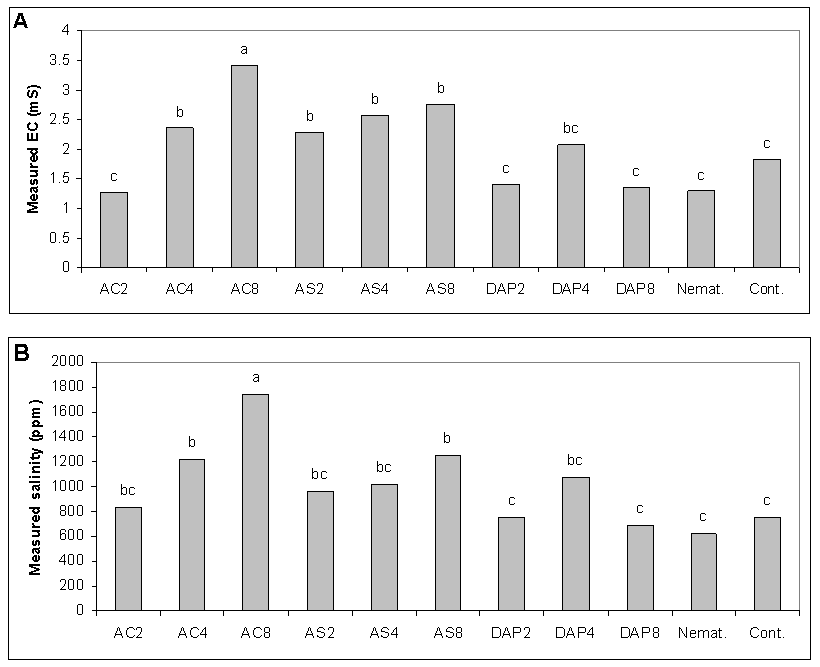
Efficacy of nitrogen salts at 2, 4, and 8 mmhos/cm electrical conductivity (EC) on the host-pathogen relationship of root-knot nematode (Meloidogyne javanica) and cucumber (Cucumis sativus) was investigated and compared with the effects of nematicides at 2 locations. Root galling of cucumber roots caused by M. javanica was more effectively suppressed using ammonium-containing salts: NH4Cl, (NH4)2HPO4 and (NH4)2SO4 at EC4 and EC8 than at EC2 and in a similar effect level of the nematicide, oxamyl or ethoprophos, and the reduction was accompanied by a reduction in the nematode final population number. At higher EC levels, NH4Cl followed by (NH4)2SO4 led to an increase of salinity up to about 1750 and 1245 mg/kg, respectively, without affecting cucumber growth and yield. Diammonium phosphate was superior over the ammonium salts in increasing cucumber growth and yield despite root-knot infection. Use of nitrogen salts could control M. javanica and improve growth and yield of cucumber under field conditions.
Keywords: Ammonium salts, Cucumis sativus, Meloidogyne javanica, Plant-nematode interaction,Management
Cite this paper: Muwaffaq R. Karajeh, Farah M. Al-Nasir, Field Utilization of Nitrogen Fertilizers for Controlling 
 Root-knot Nematode and Improving Growth and Yield of Cucumber, International Journal of Agriculture and Forestry, Vol. 4 No. 1, 2014, pp. 34-40. doi: 10.5923/j.ijaf.20140401.05.
Root-knot Nematode and Improving Growth and Yield of Cucumber, International Journal of Agriculture and Forestry, Vol. 4 No. 1, 2014, pp. 34-40. doi: 10.5923/j.ijaf.20140401.05.
1. Introduction
- Cucumber (Cucumis sativus L.) grows on most continents worldwide and is a common crop cultivated in irrigated areas of Jordan. Root-knot nematodes (RKN; Meloidogyne Goldi 1892 - RKNs) are a group of important plant-parasitic nematodes attacking higher plants[30; 33; 34], causing great crop losses[35] and reducing quality of produce[4].Three major Meloidogyne species (M. javanica [Treub] Chitw., M. incognita [Kofoid et White] Chitw., race 1 and 2 and M. arenaria [Neal] Chitw., race 2) were reported in Jordan, with M. javanica being the most widely distributed in Jordan[2; 16] causing considerable losses in yield of irrigated vegetable crops[1].Many physiological functions of the plant could be influenced by inorganic salts, especially when in an interaction with stress such as infection by nematodes[9; 32]. Some ions (NH4+, K+, NO3- and Cl-) were effective in reducing M. incognita infection of cucumber roots[6; 12; 24]. Among these NH4+, K+, and NO3- increased plant growth[6; 37]. Application of fertilizers negatively influences nematode populations indirectly by decreasing nematode feeding or by providing adequate nutrition for plants to compensate loss due to nematode feeding[26]. Nutrient deficiency could increase susceptibility of plants to nematodes[28]. Direct effects of fertilizers on nematodes may alter their behavior and reproduction resulting in changes in population[8; 17; 19]. Karajeh & Al-Nasir[18) reported that under in vitro growth chamber and greenhouse conditions, egg-hatch and second-stage juvenile viability of M. javanica were reduced when ammonium chloride (NH4Cl), ammonium sulfate (NH4)2SO4) and ammonium nitrate (NH4NO3) were used at electrical conductivity (EC) levels of 6 and 8 mmhos·cm-1 accompanied with reduction in cucumber root galling. The diammonium phosphate (NH4)2HPO4 caused the greatest reduction in root galling to <2.5 and was superior in increasing plant growth. As a salt, NaCl caused a reduction in both nematode infection and root growth especially at higher EC levels due to its salinity effect.This study investigates the effects of the nitrogen containing fertilizers; NH4Cl, (NH4)2SO4 and (NH4)2HPO4 on growth and yield of cucumber and its interaction with the RKN, M. javanica under two field conditions of Jordan.
2. Materials and Methods
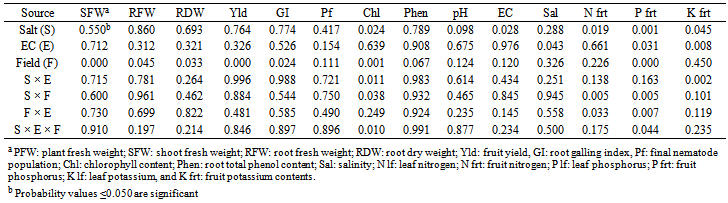
- Two M. javanica-infested fields were selected; one was located in the Ghor Safi region of southern Ghors, with semi-arid subtropical climate conditions (about 80 mm of annual rainfall and silty loam soil, at 418 m below sea level). The other was located in the Karak Valley region with semi-arid conditions (about 200 mm of annual rainfall and clay loam soil, at about 200 m above sea level) of Jordan. Drip irrigation was used on both sites. The field experiments were conducted during mid-September 2012 to mid-March 2013 and mid-February to mid-July 2013 cucumber growing seasons in Ghor Safi and Karak Valley, respectively. Field populations of RKNs of the sites were identified as M. javanica morphologically by observing the perineal patterns of females and morphometrically by measuring lengths of second-stage juveniles[5] and confirmed by Meloidogyne species-specific SCAR-PCR test[15]. Seeds of cucumber cv. Hayel were sown into holes (2 seeds per a hole) made into black plastic mulch-covered rows with 80 cm width and 150 cm spacing between rows and then irrigated. The seedling emerged after 4 days. The experiment was arranged in a randomized complete block design and each treatment (5 plants per plot) was replicated times. When plants were 2 weeks old the treatments applied using analytical reagent grade nitrogen salts were: NH4Cl, (NH4)2SO4 and (NH4)2HPO4. Electrical conductivity for each salt solution was 2 (EC2), 4 (EC4), and 8 (EC8) mmhos·cm-1 achieved by dissolving 1.067, 2.138, and 4.277 g·L-1 NH4Cl; 2.633, 5.276 and 10.553 g·L-1 (NH4)2SO4; and 2.51, 5.14, and 9.85 g·L-1 (NH4)2HPO4, respectively, in water. Nematicides Ethoprophos, 20% (Nemacap® 200EC, Chemvet, Jordan) or Vydate® 24SL (Dupont, Delaware, USA) was added to the rhizospheric soil at manufacture's recommended high application rate in Ghor Safi and Karak Valley experiments, respectively. Untreated check (control) plants were irrigated with water only. Treatments were repeated twice at 2 week-intervals from the first application for one month later. Drip irrigation, weed control, air-borne pest control and fruit harvest were according to Werner[40]. Accumulated amount of fruit yield per a treatment was recorded from 4 harvests per plots. Random samples of the fifth leaf from plant top (four leaves/ a treatment) and moderate-in-size cucumber fruits were taken at the middle of the growing season for further analyses in the laboratory. Representative random samples of oven dried leaves and fruits were finely grounded and analyzed for nitrogen, potassium and phosphorus content[29]. Chlorophyll content was determined by taking according to Hamid & Jawaid[14] with some modification (100% rather than 80% acetone was used).At the end of the experiment, 10 weeks from the date of seed germination, cucumber plants were up-rooted. Shoot and root fresh and dry weights were taken. Root galling index was evaluated according to a scale where: 0: no galling; 1: 1–2; 2: 3–10; 3: 11–30; 4: 31–100 and 5 over 100 galls[38]. The amount of total phenolics in roots was determined with the Folin–Ciocalteu reagent procedure[23; 25]. Representative rhizospheric soils were collected from treatments for nematode extraction using Baermann tray method[41] to estimate final nematode population. After sieving and grinding 10 g of oven dried rhizospheric soil was dissolved in distilled water, and soil pH, EC and salinity measured using PCSTestr35 (Eutech Instruments, Illinois, USA). Statistical analysisData were analyzed statistically using general linear model (GLM) procedure (SPSS software, ver. 11.5; SPSS Inc., Chicago, USA). Averages and analysis of variance (ANOVA) of main factors and interactions was tested at the 0.05 probability level. Least significance difference was used for mean separation.
3. Results
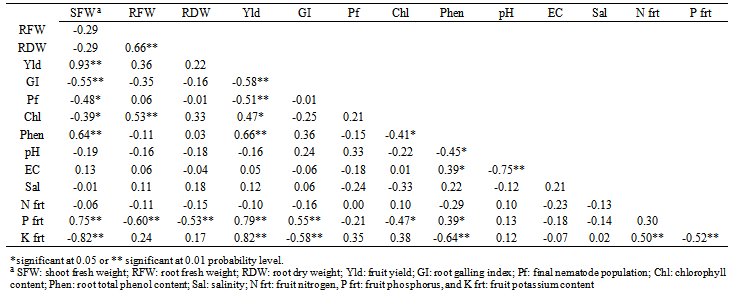
- The application of (NH4)2SO4 at EC2 and 8 and (NH4)2HPO4) at EC8 produced an increase in cucumber shoot fresh weight over the untreated control (Fig. 1A). Both (NH4)2SO4 and (NH4)2HPO4 at the higher EC produced a higher root dry weight than the untreated control (Fig. 1B). The NH4Cl; (NH4)2SO4 and (NH4)2HPO4 at EC8 and (NH4)2HPO4 at EC4 produced the highest fruit yield compared to the nematicide and the control (Fig. 1C).
4. Discussion
- Use of chemicals to control phytoparasitic nematodes is expensive, can damage the environment and suppress populations of beneficial antagonistic micro-organisms in soils. Under these field conditions, root galling of cucumber roots caused by M. javanica was better suppressed with NH4Cl, (NH4)2HPO4 and (NH4)2SO4 at in a similar manner to nematicide oxamyl or ethoprophos and the reduction was accompanied with a reduction in nematode final population. Under growth room conditions, egg-hatching and J2 viability of M. javanica were greatly reduced when NH4Cl, (NH4)2SO4 and NH4NO3 used at elevated levels of EC, and there was a reduction in rate of nematode infection after use of NH4Cl, (NH4)2SO4 and (NH4)2HPO4 but KNO3 and NH4NO3 resulted in moderate nematode infection on cucumber under greenhouse conditions[18]. Numbers of hatched eggs of M. incognita decreased with increasing ammonium concentrations by 15 days of incubation[36). Dropkin et al. (8) found that high salt concentration can modify osmotic pressure sufficiently to inhibit egg-hatching and J2 movement of Meloidogyne spp. The NaCl reduced in nematode infection and root growth, especially at higher EC levels, and could be due to salinity. High NaCl concentrations resulted in a significant suppression of M. javanica under laboratory and greenhouse conditions[35]. Under greenhouse conditions, infectivity of M. incognita was reduced at higher NaCl (EC 5) concentration than at EC 1.5[9]. At EC8, NH4Cl followed by (NH4)2SO4, increased salinity without negatively affecting cucumber growth and yield. Fertilizer components might be lethal directly to nematodes, or might alter pH and/or salinity of the soil harboring nematodes[13; 31; 39].Diammonium phosphate was best for increasing cucumber growth and yield despite root-knot infection. Symptoms of root-knot nematode infestation appears as stunted growth coupled with severe deficiency symptoms of some nutritional elements, loss of yield and a reduction in product quality[21]. Use of (NH4)2HPO4 through its nutritional effect could compensate for damage caused by the RKN. Addition of (NH4)2SO4 reduced total number of plant-parasitic nematodes; reduced root galling induced by M. incognita on cucumber; increased numbers of free-living nematodes, and resulted in increased fresh and dry weights and length of cucumber plants over the non-treated control[3]. In contrast to calcium salts that had no effect on juveniles of M. incognita, ammonium salts were strongly repellent[22]. Furthermore, the application of ammonium fertlizers was found nematicidal on other plant-parasitic nematodes (Walker, 1971; Castro et al., 1991; Sudirman and Webster, 1995; Le Saux and Queneherve, 2002). Ammonium ion may affect nematode ability to supply energy for egg-hatching and root invasion and infection (Scott and Martin, 1962; Viglierchio, 1979; Orion et al., 1980; Orion et al., 1995).Our study suggests the limited use of ammonium- containing fertilizers as a relatively safer alternative for chemical pesticides for managing the root-knot nematode effectively in those fields infested with the nematodes. Combination of ammonium amendments with solarization or a bioagent may improve their efficacy in pest management [27]. Activity against M. incognita was improved when soil solarization was used in conjunction with amendments such as ammonium phosphate or composted chicken litter[11]. Therefore, further studies are needed to investigate the effects of (NH4)2HPO4 and (NH4)2SO4 in a combination with other tactics to manage the root-knot nematode, M. javanica on cucumber.
ACKNOWLEDGEMENTS
- This work is part of a research project financially supported by the Scientific Research Support Fund of Jordan in co-ordination with the Scientific Research Deanship of Mutah University, Karak, Jordan.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML