-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2013; 3(7): 349-352
doi:10.5923/j.ijaf.20130307.14
Impact of Hydro-Priming and Osmo-Primingon Germination Indexes of Rye (Secale Cereal)
Mohsen Salami1, Davood Eradatmand Asli2, Mojtaba Yousefi Rad1
1Department of Agronomy and Plant Breeding, Islamic Azad University, Saveh Branch, Saveh, Iran
2Department of Agriculture, Payme Noor University, Iran
Correspondence to: Mohsen Salami, Department of Agronomy and Plant Breeding, Islamic Azad University, Saveh Branch, Saveh, Iran.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
This study was conducted in order to study the effect of hydro-priming by distilled water and osmopriming by KNO3 on indexes germination of Secale Cerealin triplet in factorial design and based on fully randomized plan. Factors consistto distilled water for 4 hours and KNO3 1% and 2% were composed the second factor. Germinated seeds were counted daily for eight days. Then the percent of germination, germination speed, the averagedaily germination, the length of the shoot, root and the weight of dry plant were measured. Regarding mean data resulted from the experiment the results showed that distilled water influent on the percent of germination, germination speed, the average daily germination, the weight of dry plant, the length of the shoot and the root on the level of one percent (p>0/01) significantly. In sum the results showed that hydro priming by the distilled wateris able toassistto develop suitable rye plant in real condition.
Keywords: Distilled water, Hydro priming, Germination indices, Rye
Cite this paper: Mohsen Salami, Davood Eradatmand Asli, Mojtaba Yousefi Rad, Impact of Hydro-Priming and Osmo-Primingon Germination Indexes of Rye (Secale Cereal), International Journal of Agriculture and Forestry, Vol. 3 No. 7, 2013, pp. 349-352. doi: 10.5923/j.ijaf.20130307.14.
1. Introduction
- Rye is one of the most important agricultural plants and increase in germination percent of the rye seed is an important factor in improving the plant (Kafi et al 2005). Although among the plants, rye is one of the best adjusted agricultural categories but amount of production and performance of this plant as other agricultural plants is hardly affected by environmental factors and constantly there is a concern that whether produced rye can be enough for growing population in the world (Satorr and Slafar 2001). Harris et al 2001 reported that priming the seed causes the powerful development, more rye branch fills the rye better; increase the products and length of the branches of the rye. In India the effect of priming in decreasing the development duration caused that farmers can have 3 products in a year[13]. Karaki[15] reported the increase of the wet weight and the length of the shoot and the root of the rye and barley along with priming. Determining a suitable time of priming prevents a negative effect of it. Penalosa and Eira 1993 reported that the suitable time of priming prevents a negative effect of priming on the germination seed of tomato. Seed germination has extra importance in determining the final density of the plant in the square unit so that the enough density of the plant in square unit will be achieved that cultivated seeds erupt completely with enough speed[6]. The advantages of priming the seed is reported so that include increasing the resistance of the plant in the salty areas, Asada 1992, and under dry condition[1], seed cultivation,[7], increasing the performance of the seeds with low naming power,[2], and also increase the products[11].
2. Material and Methods
- To study the effect ofdistilled water on germination indices of rye seed this experimentwas studied in terms of full randomized blocks factorial design in Plants Physiology Laboratory, faculty of Agriculture of Saveh Azad University. For this reason, rye's cultivar Secale Montanum was used, namely, first, 50 seeds were separated and disinfected for each Petri dish and in order to disinfect, the seeds were drenched in KNO3 1% and 2% for 8hours then they were washed with water. Related seeds were treat in Hydro-priming withdistilled waterfor 4 hours in 200. After this time the seeds were transferred to the sterilized pottery dish in which bottom there was a paper filter. The diagonal of all pottery dishes was 9 cm. Then, 10ml distilled water or KNO3 solutions in concentration 1% and 2% was addedafter that all of them were transferred to a germinator with 25±1 and duration of day light 16 hours and darkness was 8 hours. Light intensity was 1500 lux. Counting the germinated seeds was done daily in a specific time. In the time of counting, the seeds were considered germinated that the length of their roots was 2mm or more. Counting will be continued till the increase in the number of germinated seed won't be observed and the number of the seed in pottery dish will be fixed. According to the data, in order to calculate the percent and speed of germination, the following equation is used.Germination Percentage = S/T× 100Germination Speed = N1/D1+N2/D2+…+Ni/DiWhere s is the number of germinated seeds, T is the total seeds and Ni the number of germinated seed in day Di. The average of daily germination (MDG) = ∑Cpsgt/TWhereCpsgtis the percent of the germinated seed during the period and T is the total germination period.In order to achieve the length of the shoot and the root, 1ml ruler was used then the dry plant was measured. All samples were dried in an oven at 70°C for 72 h, and then weighed for dry matter.
3. Results and Discussion
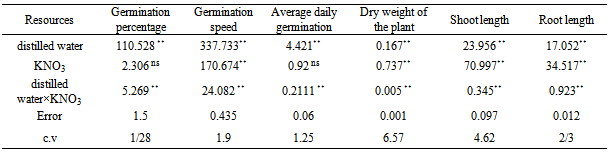
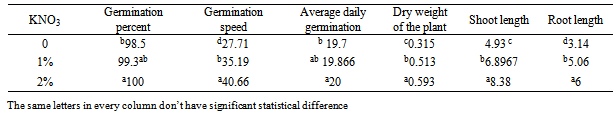
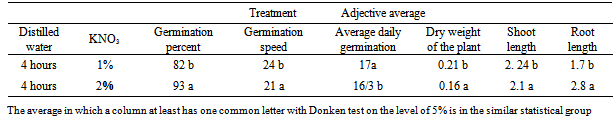
- Variance analysis results (table 1) showed that KNO3 has meaningful effect on germination percent, germination speed, the average of daily germination, dry weight of plant, the length of the shoot and the root, moreover, according to results , distilled water has significant effect on germination percent, germination speed, the average of daily germination, dry weight of plant, the length of the shoot and the root on the one percent probable level (p>0/01). interfering of distilled water with KNO3 has significant effect on germination percent, germination speed, the average of daily germination, dry weight of plant, the length of the shoot and the root on the one percent probable level (p>0/01). The results of the average of comparison effect of different level of distilled watershowed that increase of the distilled water improve the situation of germination components related to witness. The most germination indicator is related to the distilled water and KNO3 2% concentration.The results of the averages of the KNO3 (table 1) showed that the increase of KNO3 improves the situation of germination components related to the witness. KNO3 doesn’t have much effect on the germination percent and the average of daily germination. The most germination speed, dry weight, length of the shoot and the root is related to KNO3 2% treatment.
|
|
|
4. Conclusions
- According to the results in this experiment and the different level of treating the chemical KNO3 and Distilled water of this matter we can conclude that probably, KNO3 by increasing the root development and raising the ability of nutrient attraction by the plant represents this possibility in order to use the potential of the water and nutrient in the soil. The results of this research showed that seed treatment with KNO3 can be as an economic simple way and also be effective on increasing the plant output.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML