-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2013; 3(7): 333-338
doi:10.5923/j.ijaf.20130307.12
Chlorophyll Fluorescence Sorting Method to Improve Seedling Emergence Potential and Vigour of Commercial Tomato and Cucumber Seed Lots
Ibrahim Demir1, Burcu B. Kenanoglu2, Henk Jalink3, Kazım Mavi4
1Department of Horticulture, Faculty of Agriculture, University of Ankara, Ankara, 06110, Turkey
2Department of Horticulture, Faculty of Agriculture and Natural Sciences, University of Uşak, Uşak, 64200, Turkey
3Wageningen UR Greenhouse Horticulture, POB 644 NL-6700 AP Wageningen, The Netherlands
4Department of Horticulture, Faculty of Agriculture, University of Mustafa Kemal, Hatay, 31000, Turkey
Correspondence to: Burcu B. Kenanoglu, Department of Horticulture, Faculty of Agriculture and Natural Sciences, University of Uşak, Uşak, 64200, Turkey.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
This work was conducted to investigate whether chlorophyll fluorescence (CF) sorting enhanced the seedling potential and vigour of commercial seed lots of tomato (Solanum lycopersicum Mill) and cucumber (Cucumis sativus L.). Eight seed lots (4 each in 2008 and 2009) for each species were sorted and laboratory germination, seedling emergence tests were conducted. Results indicated that CF sorting did not significantly increase laboratory germination. However, its effect was more prominent on emergence potential (p<0.05). It can be concluded that CF as a non-destructive sorting technology has the potential to upgrade seed quality.
Keywords: Tomato, Cucumber, Seedling Emergence, Chlorophyll Fluorescence
Cite this paper: Ibrahim Demir, Burcu B. Kenanoglu, Henk Jalink, Kazım Mavi, Chlorophyll Fluorescence Sorting Method to Improve Seedling Emergence Potential and Vigour of Commercial Tomato and Cucumber Seed Lots, International Journal of Agriculture and Forestry, Vol. 3 No. 7, 2013, pp. 333-338. doi: 10.5923/j.ijaf.20130307.12.
1. Introduction
- The high initial cost of vegetable crop seeds, i.e. hybrids, has led to growers to employ precision seedling and transplant production systems. High seedling stands have become a necessity for saving cost and time. At the same time, high quality seeds have become more reliable with regard to maximal emergence and uniformity. The physiological quality of a seed lot is the result of pre-storage factors, the acquisition of the ability to produce vigorous seeds (optimum maturation) and post-storage factors[22].Vegetable species such as tomato and cucumber have a continuous flowering behaviour which causes the occurrence of variously matured seeds on the plant at the same period. This is very likely to happen in once-over harvesting systems, i.e. when harvesting differently matured fruits at once. Gradual harvesting, i.e. harvesting earlier maturated fruits separately from less mature ones, may eliminate some differences in the lot. However, this is not easy, and is expensive to apply in large scale commercial production. Therefore, fruits that contain seeds at different maturation levels in some vegetables such as tomatoes and cucumbers due to differences in flowering time, fertilization, cluster position, seed position in the fruit, and radiation level are harvested at once and produce a single lot. In such harvests, the seed lot consists of a mixture of less mature and fully mature seeds. Seed-to-seed variation in the maturation of a single lot results in lower uniformity and seedling size during transplant production because less mature seeds germinate more slowly and produce smaller seedlings while mature seeds emerge faster and produce larger seedlings[7]. Moreover, less mature seeds are likely to show physiological ageing earlier during the post-harvest storage period[8], since they are more prone to ageing particularly when storage conditions are non-optimum. Therefore, separating less mature seeds from the lot would have the potential to enhance the overall quality of a seed lot.One of the basic tenets of maturation is decomposition of the chlorophyll content of the seed coat as the seed matures[24]. There was close relationship between seed coat chlorophyll content and maturation level in oilseed rape and turnip seed[26, 27]. Less mature seeds are distinguished by high chlorophyll content while matured ones have a lower chlorophyll content. Various seed sorting methodologies based on physical and chemical properties have been developed[5, 3, 11]. One of them is a non-destructive technique for assessing the maturity of seeds, relying on measuring the amplitude of the chlorophyll fluorescence (CF) signals of intact seeds. The technique makes use of laser technology, narrow optical bandwidth filters, and detection of chlorophyll a in the seed coat, measuring the resulting chlorophyll fluorescence and linking it to the quality of seeds in cabbages[14, 6], tomatoes[15], barley[19] and carrots[11]. These studies assessed seed quality based on germination tests. However, improving the seedling emergence potential of a seed lot is important for crops that are produced by transplantation such as cucumber and tomato. These crops are produced through transplants and high stand establishment and uniformity is valuable, particularly for off-season glasshouse production. Germination percentage is a basic component of seed quality. It does not always indicate the emergence potential of a lot since further quality assessment features such as seed vigour level is involved in seedling emergence, in which adverse soil properties and the environment are involved in the overall performance. In this work, we investigated to what extent CF sorting enhanced laboratory germination, seedling emergence percentages of commercially available tomato and cucumber lots.
2. Material and Methods
- Eight seed lots each of tomato (Solanum lycopersicum cv. Falcon) and cucumber (Cucumis sativus L. cv. Beith Alpha) were obtained from various commercial seed companies in 2008 and 2009. Specific attention was paid to the seed lots to ensure that they were produced in the same year. Thus, seed lots used in the experiment were produced in two different years and by different companies. This aimed to test the methodology for seed lots from various sources. Initial seed moisture contents were determined according to ISTA (2004), and ranged between 6.1 and 7.2% in tomatoes and 6.7 and 8.5% in cucumbers. Initial laboratory germination tests were conducted on three replicates of 50 seeds each lots. The seeds of each replicate were placed between three moistened 20 x 20 cm filter papers (Filtrak, Germany), two below and one above, which were wetted with 6 ml of distilled water each. These papers were then rolled up and placed in plastic bags in order to prevent water loss. Germination tests were carried out at 25℃ in the dark. Total (radical protrusion) and normal (developed shoot and root structures) seedlings were evaluated after 14 days in tomatoes and 8 days in cucumbers. CF sorting was done using the methodology developed at Plant Research International in Wageningen, The Netherlands[14]. One thousand seeds in each lot were sorted below 730 nm, approximately 60% in tomatoes, below 1030 nm 65% in cucumbers and designated as CF sorted. Non-sorted seeds were considered as controls. Then 16 seed lots for each species (8 sorted/8 control) were all tested for laboratory germination, seedling emergence in modules, and controlled deterioration vigour tests. All tests were conducted within 20 days after sorting, and during that period seeds were kept at 5℃ in hermetically sealed aluminium foil packets. Laboratory germination tests were conducted (three replicates of 50 seeds) as described above on CF sorted and control seed lots. Emergence tests were conducted with three replicates of 50 seeds in CF sorted and control seeds. Seeds were sown 2 cm deep in compost (Plantaflor, Humus Verkaufs, GmBH, Germany). Seedlings were grown in a growing cabinet at 20±2℃ for 16 days. Light was provided by cool fluorescent lamps (Philips) at a rate of 78 μmol. m-2.s-1. for 12 h d-1 on the seedling level. Relative humidity in the cabinet was maintained above 70% throughout the experiment to minimise water loss from the boxes. Watering was done with an equal amount of water and at the same time of the day. In the growing cabinet, boxes were rotated every day to obtain uniform temperature during emergence. Appearance of the hypocotyl hook on the compost surface was used as an emergence criterion, counted daily at the same time of the day, and finally total and normal emerged seedlings were recorded as percentages.The mean germination (MGT) and emergence time (MET) were calculated for CF sorted and control lots of each species during germination, emergence and following controlled deterioration tests by the formula cited by Ellis and Roberts (1980) given below:
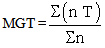 wheren = number of seeds newly germinated / emerged at time T,T = days from the beginning of the germination / emergence test,Σ n = final germination / emergence. Statistical analysis was performed using SPSS to carry out Duncan. Means of CF sorted and control seed lots in each species were compared at 5% level. Percentages were arcsine transformed prior to analysis.
wheren = number of seeds newly germinated / emerged at time T,T = days from the beginning of the germination / emergence test,Σ n = final germination / emergence. Statistical analysis was performed using SPSS to carry out Duncan. Means of CF sorted and control seed lots in each species were compared at 5% level. Percentages were arcsine transformed prior to analysis. 3. Results
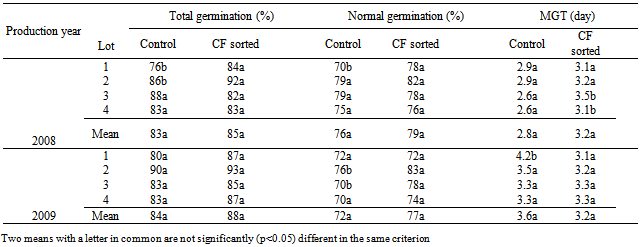
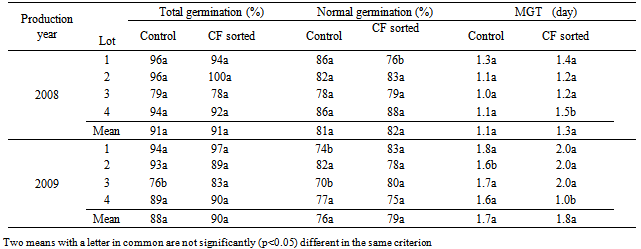
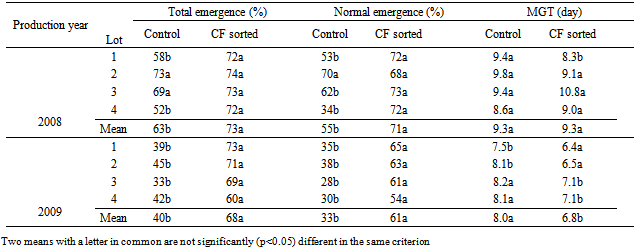
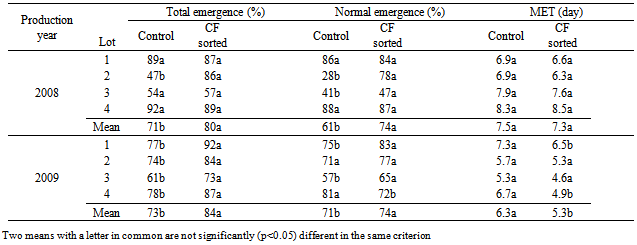
- CF sorting did not significantly increase total normal germination and mean germination times in laboratory germination tests in both species and years. There were some individual seed lots in which CF sorting increased germination percentages significantly, such as the total germination of seed lot 1 in tomatoes of 2008 and the normal germination of lot 1 in cucumbers of 2009 (Table 1). However, when the means of five lots in each year were compared, CF sorted seed lots either had the same percentages or slightly higher but not significant values (P<0.05). The advantage of CF sorting as laboratory germination percentages ranged between 2 and 4% in tomato, and 0 and 4% in cucumber seed lots (Table 1 and 2). Regarding mean germination time (MGT), there was no remarkable difference between CF sorted and control seed lots. In some cases CF sorted seeds had longer MGT values, i.e. tomato and cucumber seed lots in Mean 2008, but this was not significant. CF sorting affected significantly (P<0.05) the total and normal tomato and cucumber seedling emergence percentages (Tables 3 and 4). The greatest advantage, 23%, was observed in total and normal seeding percentages of 2009 seed lots in tomatoes. This was 8% in total and 14% in normal seedlings in 2008. The mean germination time of tomato seed lots was not significantly affected by CF sorting (P>0.05) in 2008 but was in 2009. In general, cucumber seed lots had higher emergence percentages than those of tomatoes. CF sorting increased the total germination from 76 to 84% in 2008, and 70 to 86% and 2009. Corresponding values in normal cucumber seedling emergence percentages were from 68 to 79% and from 67 to 78%. CF sorted cucumber seeds emerged earlier than the controls. This was variable among the lots but with regard to mean MGT values, the advantage was 0.3 days in 2008 and 1.0 days in 2009.
|
|
|
4. Discussion
- Results of the present paper indicated that CF sorting increased seedling emergence percentages. However, the sorting effect was little and non-significant for laboratory germination. These conclusions are in agreement with findings of previous papers on tomatoes[15], cabbages[14], barley[19] that the CF method has the potential to enhance seed quality. However, these studies evaluated seed quality based on laboratory germination which is normally conducted under optimum germination conditions. In our work we tested the effect of CF sorting not only on laboratory germination but also on emergence as seed performance. The CF sorted seeds showed slightly better but not statistically significant germination compared to the controls, while CF sorting enhanced seedling emergence potential and separated less aged seeds in both species significantly (p<0.05), (Tables 1, 2, 3 and 4). Transplant production and quality are important for horticultural crop seeds. Extended use of high value seeds in vegetable species such as hybrids necessitates production of a plant for each seed in a seed lot. This is also the case in tomatoes and cucumbers. Ideally, every single seed is expected to produce a healthy, strong seedling. Transplant production is conducted under controlled conditions in which high financial input (heating, planting medium, etc.) and workforce are necessary. Thus, every non-germinated seed is a waste of money and time. Moreover, timely production in off-season growing in glasshouse conditions requires a start with well developed seedlings to get the maximum yield on time. Various methodologies are used to sort seeds and improve the quality, i.e. various image analyses and priming. However, CF sorting has the unique feature of non destructiveness. During CF there is no germination requirement and no imbibition occurs in the seed. Following imbibition, the seed becomes sensitive to drying and subsequent storage[25]. CF is well suited to vegetable crops in which seed maturation occurs gradually due to continuous flowering and fertilization[7, 21, 8] on the mother plant. So variation within the same lot is more likely to be higher than in field crops. CF is a non-destructive technique for assessing the maturity of seeds, relying on measuring the amplitude of the chlorophyll fluorescence (CF) signals of intact seeds. However, there are two basic cautions about CF. On is that it may be used on species such as aubergine, maize, and sunflowers in which there is little or no chlorophyll in the seed coat which can be detected over maturation. One other is that when a large proportion of seeds are discarded due to the high CF value, these seeds are considered as loss. In this way, the financial effort and expense for these seeds become extra expenses for production. In this experiment we discarded 25 and 35 % of the seeds out of the lot. This is quite a high proportion. But it is associated with the pre-sorting maturation level of the seed lot. The more immature seeds in the lot, the more seeds are eliminated. This may be alleviated through high quality seed production at the first place, i.e. harvesting at maximum maturity, and this will reduce the proportion of low quality seed in the lot. Moreover, its use may be debatable in cases where seed coat chlorophyll content or colour may change dramatically among cultivars within the same species, as in Brassica napus[28]. CF sorting was found to be more effective on emergence percentages and separating seeds based on physiological ageing level. Germination is an important component of seed quality but it is tested under optimum conditions in which even low quality seeds may have ability to germinate. However, when sowing conditions for seedling production are non-optimum, which is a common phenomenon in early spring sowings, low quality seeds are not able to perform as well as those of high quality which show a higher level of seed vigour. High quality seeds emerge earlier and produce larger seedlings in adverse conditions. Low CF value seeds appeared to emerge earlier as indicated by mean emergence time (Table 3 and 4). An extended period of the seedling in the seed bed, as is the case with deep sowing, may increase the risk of infection by ‘damping-off’ pathogens such as Fusarium, Rhizoctenia and Pythium. The enhanced and more rapid seedling emergence provided by CF sorting reduces the time that seedlings are covered in the seed bed, thereby alleviating the possibility of pathogenic attack. Less mature seeds are likely to leak solutes to the seed bed. These solutes attract fungi and bacteria which consume oxygen in the seed bed and make it impossible for the seedling to emerge. This is in agreement with the findings of Konstantinova et al. (2002) which indicated that CF sorting helped to eliminate those seeds that were more likely to get infected in barley. Indeed lower vigour and shorter seed longevity may be associated with high chlorophyll content of seeds. Recently the strong photo-oxidation process that occurs during storage in Salix seeds was shown to be accounted for by their high chlorophyll content[23]. Pre-harvest ecological factors influence seed maturation[1, 2]. We selected 8 lots in each species 4 of them produced in 2008 and the other four in 2009. Even though they were produced in the same year, the lots came from different seed companies and were produced in various parts of the country (i.e. with different environmental features during growing). This may cause differences in pre-marketing seed quality of the lots. Obtaining similar results in both years particularly regarding seedling emergence confirmed unanimously that CF worked out well in seed lots produced in various environments in two subsequent years. Differences in individual seed lots in the same year or between years may also result from different harvesting practices such as extraction, drying, or fruit maturation time which resulted in variable amount of less mature seeds in the lot. These factors also affect the ageing level of any seed lot[21]. The effect of CF sorting not only on laboratory germination, but also on emergence and physiological aging as indicators of seed vigor. Especially, in pepper seed lots CF sorting significantly increased laboratory germination, seedling emergence, and seed vigor[18].In conclusion, CF can be a reliable tool to separate high quality seeds (i.e. more mature) from low quality (i.e. less mature) seeds within once-over harvested tomato and cucumber seed lots. This may be usable for improving seedling production potential and separating less vigorous (less aged) seeds from the lot thus increasing the overall vigour of the lots.
ACKNOWLEDGMENTS
- We thank Ankara BAP-Pro (Ankara University, Scientific Research Project) for their financial support.
References
| [1] | Abdul-Baki, A.A. and Anderson, J.D. 1972. Physiological and biocemical deterioration of seeds. Seeds Bology (Ed. by Kozlowski; T.T.), Academic Pres, 2:283-315. |
| [2] | Bewley, J.D. and Black, M. 1982. Viability, dormancy and environmental control. ‘In ‘Physiology and Biochemistry of Seed in Relation to Germination’ vol.2, Springer-Verlag, Germany, 375 p. |
| [3] | Chen, P. and Sun, Z. 1991. A Review of non-destructive methods for quality evalution and sorting of agricultural products. Journal of Agricultural Engineering Research, 49, 85-98. |
| [4] | Dell’Aquila et al., 2000. The application of image analysis in monitoring the imibibition process of white cabbage seeds. Seed Science Research, 10,163-169. |
| [5] | Dell’Aquila, A. 2007. Towards new computer imaging techniques applied to seed quality testing and sorting. Seed Science and Technology 35, 519-538. |
| [6] | Dell’Aquila, A., van der Schoor, R. and Jalink, H. 2002. Application of chloropyhll fluoresence in sorting controlled deteriorated white cabbage seeds. Seed Science and Technology 30, 689-695. |
| [7] | Demir, İ. and R. H. Ellis. 1992. Development of pepper seed quality. Annals of Applied Biology. 121: 385-399. |
| [8] | Demir, İ. 2002. The effect of controlled hydration treatment on germination and seedling emergence of unaged and aged pepper seeds during development. Israel Journal of Plant Sciences. 50: 251-257. |
| [9] | Ellis, R.H. ve Roberts, E.H. 1980 Improved Equations for the Prediction of Seed Longevity. Annals of Botany, 45, 13-30. |
| [10] | Geneve, R.L. and Kester, S.T. 2001. Evaluation of seedling size following germination using computer- aided analysis of digital images from a flat-bed scanner. HortScience 36, 1117-1120. |
| [11] | Groot, S.P.C., Y. Birnham, N. Rop, H. Jalink, G. Forsberg, C. Kromphardt, S. Werner, and E. Koch. 2006. Effect of seed maturity on sensitivity of seeds towards physical sanitation treatments. Seed Science and Technology 34:403–413. |
| [12] | Harrington, J.F. 1973. Biochemical basis of seed longevity. Seed Science and Technology, 1: 453-461. |
| [13] | ISTA 2004. International rules for seed testing. Zurich, International Seed Testing Association. 358 p. |
| [14] | Jalink et al., 1998. Chlorophyll fluorescence of Brassica oleracea seeds as a non-destructive marker for seeds maturity and seed performance. Seed Science Research, 8, 437-443. |
| [15] | Jalink et al., 1999. Seed chlorophyll content as a indicator for seed maturity and seed quality. Acta Horticulturae, 504, 219-227. |
| [16] | Jalink, H., van der Schoor, R., Schapendonk, A., 2004. A method and a device for making images of the quantum efficiency of the photosynthetic system with the purpose of determining the quality of plant material and a method and a device for measuring, classifying and sorting plant material. Dutch Patent No. 1,021,800. |
| [17] | Jing, H.C., Bergervoet, J. H. W., Jalink, H., Klooster, M., Du, S. L., Bino, R. J., Hilhorst, H. W. M. And Groot, S.P.C. 2000. Cucumber (Cucumis sativus L.) seed performance as influenced by ovary and ovule position. Seed Science Research, 10, 435-445. |
| [18] | Kenanoglu, B.B., Demir, I. and Jalink, H. 2013. Chlorophyll fluorescence sorting method to ımprove quality of capsicum pepper seed lots produced from different maturity fruits. HortScience, 48(8): 965-968. |
| [19] | Konstantinova et al., 2002. Chlorophyll fluorescence sorting as a method for improvement of barley seed health and germinaton. Seed Science and Technology. 30, 411-421. |
| [20] | Kwong, F. Y. 1991. Research needs in the production of high quality seeds. In. Prakash, J. And Pierek R. L. M. (Eds.) Horticulture, New Technologies and Applications, Kluwer Academic Publishers, Dordrecht, pp.13-20. |
| [21] | McDonald M.B. 1999. Seed deterioration: physiology, repair and assesment. Seed Science and Technology 27: 177-237 |
| [22] | Powell, A.A., S. Matthews, and M.A. Oliveira. 1984. Seed quality in grain legumes. Adv.Appl. Biol. 10:217–285. |
| [23] | Roqueiro, G., Facorro, G. B., Huarte, M. G., Celis, E. R., Garcia, F., Maldonado, S., and Maroder, H. (2010). Effects of photooxidation on memmbrane integrity in Salix nigra seeds. Annals of Botany 105, 1027-1034. |
| [24] | Steckel, J.R.A., Gray, D.,and Rowse, H.R. 1989. Relationships between indices of seed maturity and carrot seed quality. Annals of Applied Botany 114, 177-183. |
| [25] | Tarquis AM, Bradford KJ. Prehydration and priming treatments that advance germination also increase the rate of deterioration of lettuce seeds. Journal of Experimental Botany.1992;43:307–317 |
| [26] | Ward, K., R. Scarth, J. Daun, and P.B.E. McVetty. 1992. Effects of genotype and environment on seed chlorophyll degradation during ripening in four cultivars of oilseed rape (Brassica napus).Can. J. Plant Sci. 72:643–649. |
| [27] | Ward vd. 1995. Chlorophyll degradation in summer oilseed rape and summer turnip rap during seed ripening. Canadin Journal of Plant Science, 75; 413-420. |
| [28] | Zhang, W. and Gusta, L.V. (2010). Germination response of black and yellow seed coated canola lines to chemical treatments under cold temperature conditions. Plant Growth Regulation 60, 105-114. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML