Elsadig B. Ibrahim1, Abdel Wahab H. Abdalla2, Elshiekh A. Ibrahim3, Ahmed M. El Naim3
1Department of Crop Sciences, Faculty of Natural Resources and Environmental Studies, Peace University, P.O. Box 20, El Fulla, Sudan
2Department of Agronomy, Faculty of Agriculture, University of Khartoum, Sudan
3Department of Crop Sciences, Faculty of Natural Resources and Environmental Studies, University of Kordofan, P. O. Box 160, Elobeid, Sudan
Correspondence to: Ahmed M. El Naim, Department of Crop Sciences, Faculty of Natural Resources and Environmental Studies, University of Kordofan, P. O. Box 160, Elobeid, Sudan.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Abstract
Sixteen genotypes of Roselle (Hibiscus sabdariffa) were evaluated at Shambat (Sudan) for two consecutive seasons to assess genetic variability and heritability of different characters. Field experiment was conducted at Shambat Demonstration Farm in a randomized complete block design with three replications. Data were collected on thirteen plant attributes. Analysis of variance indicated significant differences among the sixteen genotypes for most of the characters studied. In general, the morphological characters had larger environmental variances than their respective genotypic ones. The highest genotypic coefficient of variation was exhibited by the seed yield/plant in both seasons. The highest genetic advance, 46.63 % in the first season and 170.13 % in the second season were obtained both for calyx yield/unit area. High values of heritability estimates (> 0.70) were recorded for days to 50 % flowering over the two seasons. On the other hand, the yield components showed low heritability estimates (< 0.50) except for fruit weight in the second season.
Keywords:
Roselle, Karkade, Hibiscus Sabdariffa, Variability, Heritability, Sudan
Cite this paper: Elsadig B. Ibrahim, Abdel Wahab H. Abdalla, Elshiekh A. Ibrahim, Ahmed M. El Naim, Variability in Some Roselle (Hibiscus sabdariffa L.) Genotypes for Yield and its Attributes, International Journal of Agriculture and Forestry, Vol. 3 No. 7, 2013, pp. 261-266. doi: 10.5923/j.ijaf.20130307.02.
1. Introduction
Roselle (Hibscus sabdariffa L.) is a tetraploid (2n = 4x = 72) where the chromosomes are more related to the diploid (2n = 2x = 36) Hibiscus canabinus[1]. It is highly self pollinated member of the family malvaceae. Two botanical types of roselle, namely H. sabdariffa var. altissma and H. sabdariffa var.sabdariffa The first grown for its phloem fiber and the second for its fleshy, shiny-red calyxes which are usually extracted in hot or cold water and consumed as a beverage. Roselle in Sudan gaining importance in the manufacture of many small industries, e.g. cosmetics, sweets, sauces, jams, and jellies and a substitute for tea and also used as a coloring material for food. It is also used in medicine, especially with problems related to the digestive tract[2] Roselle is an important annual crop which grows successfully in the tropics and sub-tropics[3],[12].In Sudan, roselle (locally knowon as Karkade) is grown extensively in Darfor and Kordofan States under rainfed conditions[4]. Where large quantities are produced for local consumption and export purposes. Central Bank of Sudan[5] reported that the total exported quantities of dry calyxes of roselle were 18531 and 15656 tons with total income 17.59 and 14.09 million US dollars in 2011 and 2012, respectively.In spite of its potential economic importance, Karkade has received little attention. Information regarding breeding, genetics and production of karkade is meager. The cultivars used for production in Sudan are local types, which are, characterized by low yield potential. Therefore this study aimed to evaluate sixteen genotypes for yield and yield components.
2. Materials and Methods
The plant materials used in this study consist of sixteen inbred lines of Roselle (Hibiscus sabdariffa var. sabdariffa), which were derived by single plant selection. These lines differ mainly in capsule shape, plant height, leaf shape and color of the calyx, stem and petiole color, number of branches at the base of the stem and type of foliage. The material was planted in heavy cracking clay soil of the Demonstrating Farm, Faculty of Agriculture, University of Khartoum (latitude 15° 40′ N and 3° 32′ E and 376 meter above sea level) for two seasons, namely, 1998/99 and 1999/00. A randomized Complete Block Design (RCBD) with three replications was used in each season to laying out the field experiment. The gross plot size was 3x3 m2, consisting of four ridges 70 cm apart. The spacing was 60 cm between holes along the ridge. Five or four seeds were sown per hole on the shoulder of the ridge. Sowing was on the 14th of July in the first season and 12th July in the second season. Three weeks after sowing, the plants were thinned to three per hole. The experimental plots were irrigated at an average interval of 12-14 days in both seasons, with a total of eight and nine irrigations for the first and second seasons, respectively. Nitrogen fertilizer at the rate of 40 kg N/feddan was applied, three weeks after sowing. Manual weeding was carried out three times during each growing season. In both seasons data were collected for: days to 50 % flowering, plant height (cm), number of fruiting branches/plant, number of capsules/branch, number of capsules/main stem, mean calyx weight/capsule (g), calyx yield/plant (g), calyx yield/unit area, number of seeds/capsule, 1000- seed weight (g), fruit weight (g), fruit yield/plant (g), seed yield/plant (g).Individual analysis of variance was carried out for each season separately according to Gomez and Gomez[6] Then the combined analysis of variance was done as described by[7] Tables 1 and 2 show, respectively, the forms of individual analysis of variance and the combined analysis of variance.Table 1. The form of analysis of variance and expected mean square (EMS) in a randomized complete block design (RCBD) used in the study of the extent of variability for 16 Roselle genotypes
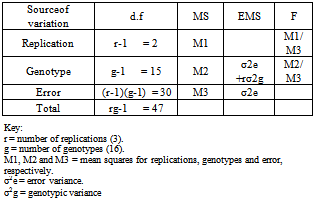 |
| |
|
Table 2. Form of the combined analysis of variance and expected mean square for pooled data of the two seasons
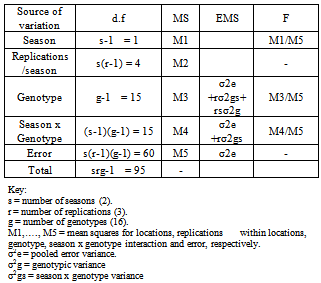 |
| |
|
Coefficient of variation (CV %) for each character in each season was determined using the formula: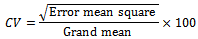 Phenotypic (σ²ph) and genotypic (σ²g) variances were estimated using individual analysis of variance (Tables 1 and 2) as follows:
Phenotypic (σ²ph) and genotypic (σ²g) variances were estimated using individual analysis of variance (Tables 1 and 2) as follows: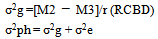 Genetic coefficient of variation (GCV %) was computed following Burton and De Vane[7] as:
Genetic coefficient of variation (GCV %) was computed following Burton and De Vane[7] as: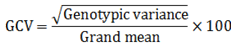 Heritability (h²) in broad sense was estimated for each character using the following procedure:
Heritability (h²) in broad sense was estimated for each character using the following procedure: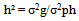 Expected genetic advance under selection (GA) was estimated following Allard[17] Then, the estimated GA was expressed as percentage of the overall mean of the character:
Expected genetic advance under selection (GA) was estimated following Allard[17] Then, the estimated GA was expressed as percentage of the overall mean of the character: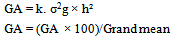 Where:k = the selection differential, and it equals 2.06 for 5% selection intensity
Where:k = the selection differential, and it equals 2.06 for 5% selection intensity
3. Results and Discussion
The assessment of phenotypic and genotypic variability is of great importance for using any efficient selection method in order to improve a population. This is because selection of favorable genotypes for certain characters depends on the amount of variation existing in the material under investigation. Such variation can be assessed by estimating its different components. In addition, the relative magnitude of these components determines the genetic properties of population. This is accomplished by estimating the role of heredity versus environment in determining the phenotype.In the present investigation, the sixteen genotypes exhibited significant differences for most of the traits studied over the two seasons (Table 3). The observed differences among these genotypes can be attributed to genetic as well as to environmental factors. Similar results had been reported by many workers. In addition, the combined analysis of variance (Table 4) revealed highly significant differences among the evaluated genotypes for most of the studied characters. Ibrahim and Hussein[9]detected significant differences among genotypes for plant height, number of branches, seed weight and sepals dry weight. The overall means of some traits showed differences between the two seasons (Table 5). This change in the overall mean for these traits was due to the interaction of the genotypes with the environment. This reveals that the observed differences among genotypes in these traits can be attributed to genetic causes as well as their interactions with the environment.Estimates of genetic variances were higher than their respective environmental ones for days to 50 % flowering and 1000-seed weight in the two seasons; plant height, number of fruiting branches/plant, fruit weight and number of seeds/capsule in one season (Table 6). This result reveals that a large proportion of the phenotypic variance for such traits was due to genetic effects, so selection for these traits will be effective.In general, the morphological characters had larger environmental variances than their respective genotypic ones. However, the influence of the environmental factors on the expression of other characters as indicated by the magnitude of the environmental variance was quite evident. For number of capsules/main stem, number of capsules/branch, mean calyx weight, fruit yield/plant, seed yield/plant, calyx yield/plant and calyx yield/unit area estimates of environmental variance were greater than their respective genotypic ones in both seasons (Table 6). This indicates that a large proportion of phenotypic variance was due to environmental causes, hence such characters do not possess promising genetic variation; therefore, selection for them will not be effective and solely would be very low. Table 7 shows the results of genotypic coefficient of variation (GCV %), heritability (h2) and expected genetic advance under selection (GA %) for the different traits. Genetic coefficient of variation, heritability estimates, and genetic advance for most of the characters were greater in the second season than in the first one. This difference could be attributed to the lesser effect of the environmental factors on these traits in the second. The amount of genetic variability is a major determiner of the genetic gain from selection. In this study, the characters showed a wide range of genetic variability among the evaluated genotypes[21],[22]. The highest genotypic coefficient of variation was exhibited by the seed yield/plant (19.7 and 27.9 in the first and second seasons, respectively), whereas the lowest value (3.32 %) was scored for days to 50 % flowering in the first season and (5.07 %) for number of capsules/main stem in the second season. The highest genetic advance, 46.63 % in the first season and 170.13 % in the second season were obtained both for calyx yield/unit area. On the other hand, the lowest genetic advance (0.04 %) was scored by mean of calyx weight/capsule in the first season and (0.20 %) in the second one. This result indicates that the genetic advance from selection for a trait depends on the amount of genetic variability of such traits. Similar conclusions have been drawn by[10]).Falconer[11] concluded that more variable condition reduce heritability, whereas uniform conditions increase it. High values of heritability estimates (> 0.70) were recorded for days to 50 % flowering over the two seasons, and 1000-seed weight in the first season, indicating that these characters possessed a wide range of genetic variability and their improvement could be achieved with mass selection alone. These high estimates of heritability could be attributed to the difficulty of separation of all genotype and environment interactions from genotypic variance since the study was carried out in one location and thus the heritability estimates were biased upward. On the other hand, the yield components showed low heritability estimates (< 0.50) except for fruit weight in the second season. The low heritability for calyx yield was due to the fact that it depends on many components which are greatly influenced by environment. Similar results were pointed out by [13],[18],[19],[20].Table 3. Mean squares from analysis of variance for different characters of 16 Roselle (Hibiscus sabdarifffa) genotypes evaluated at Shambat in two seasons
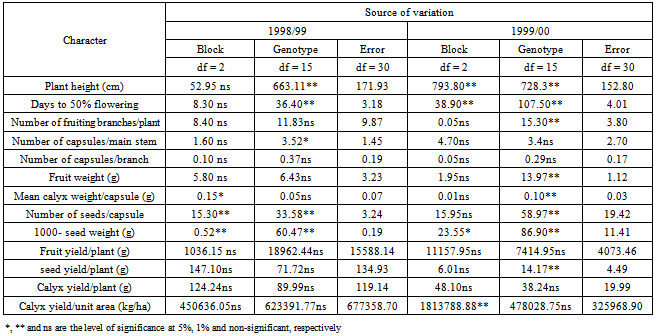 |
| |
|
Table 4. Mean squares from the combined analysis of variance for different characters of 16 Roselle (Hibiscus sabdarifffa) genotypes evaluated at Shambat in two seasons
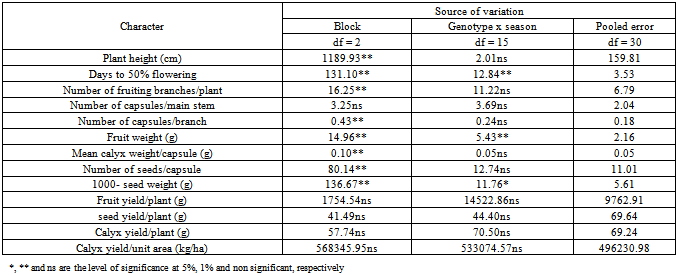 |
| |
|
Table 5. Mean, range and coefficient of variation (CV%) for the different characters of 16 Roselle (Hibiscus sabdarifffa) genotypes evaluated at Shambat in two seasons
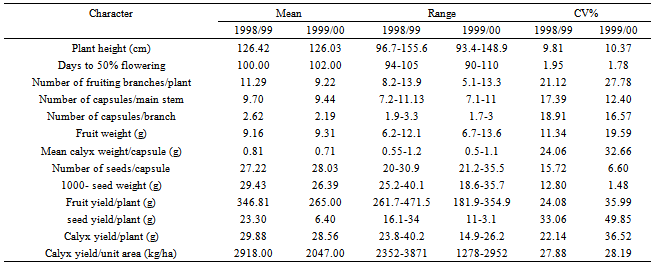 |
| |
|
Table 6. Phenotypic (σ2ph), genotypic (σ2g) and environmental (σ2e) variances for the different characters of 16 Roselle (Hibiscus sabdarifffa) genotypes evaluated at Shambat in two seasons
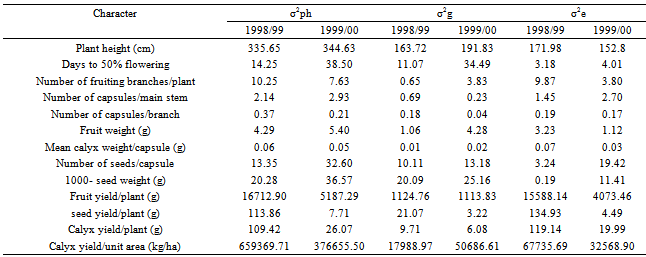 |
| |
|
Table 7. Estimates of genetic coefficient of variation (GCV%), heritability (h2) and expected genetic advance from selection (GA) for the different characters of 16 Roselle (Hibiscus sabdarifffa) genotypes evaluated at Shambat in two seasons
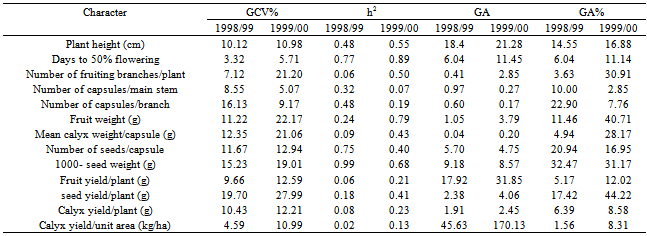 |
| |
|
However, the association of the genetic advance and heritability does not follow the same pattern as that between genetic advance and genotypic coefficient of variation. Increase in heritability value was not always accompanied with increase in genetic advance. Similar results were obtained by Gasim and Khidir[14], but high heritability with high genetic advance were observed in plant height, whereas low heritability with low genetic advance were detected in number of capsules/branch and mean calyx weight/capsule. The nature of association between heritability and genetic advance was explained by Panse[15], who reported that the association of high heritability with a high genetic advance is an indication of additive gene effects and consequently a high genetic gain from selection could be expected. On the other hand, the association of low heritability with low genetic advance is an indication of non-additive gene effects and consequently, a low genetic gain, would be expected from selection.However, Johnson et al.[16] stated that heritability does not provide an actual measurement of the amount of genetic variation, as the magnitude of heritability depends on the degree of association between the genotypic and phenotypic variances regardless of being high or low, while the genetic gain depends on the amount of genetic variability. Similar ideas were expressed by Allard[17].
4. Conclusions
Wide genetic variability detected in the tested Roselle Genotypes. This variability exploited in different breeding programs.There was no definite trend between genetic coefficient of variation and heritability or between the later and genetic advance. Therefore, conjunction of heritability estimates with genetic advance in a selection program is essential.Since calyx yield/plant showed low heritability, the indirect selection through its components assumes important.
References
| [1] | Wilson, F. D. and Menzel, M. Y. (1964). Kenaf (Hibiscus cannabinus), roselle (Hibiscus sabdariffa L.). Economic Botany, 18 (1): 80-91 |
| [2] | El Naim, A M, Khaliefa, E. H., Ibrahim, K A, Ismaeil, F. M, Zaied, M M B. 2012. Growth and Yield of Roselle (Hibiscus sabdariffa L) as Influenced by Plant Population in Arid Tropic of Sudan under Rain-fed. International Journal of Agriculture and Forestry., 2(3): 88-91 |
| [3] | Cobley, L. S. 1975. An Introduction to Botany of Tropical Crops. Longman group, U.K |
| [4] | El Naim, A. M. and Ahmed, S. E.. 2010. Effect of weeding frequencies on growth and yield of two roselle (Hibiscus sabdariffaL) Varieties under rain fed. Australian Journal of Basic and Applied Sciences, 4(9): 4250- 4255 |
| [5] | Central Bank of Sudan. 2012. Foreign Trade Statistical Digest (October-December 2012), page 12. Central Bank of Sudan, Khartoum Sudan. |
| [6] | Gomez, K. A. and A. A. Gomez. 1984. Statistical Procedures for Agricultural Research. 2nd . Ed. John Wiley and Sons, Inc. New York. |
| [7] | Le Clerg, E.L.; Leonard, W.H. and Clark, A.G. 1962. Field Plot Techniques. 2nd ed., Burgass Publishing Co.,Minneapolis, Minnesota. |
| [8] | Burton, G.W. and De Vane, E.M. 1953. Estimatingheritability in tall fescue (Fescuea arundianaceae L.) from replicated clonal material. Agronomy Journal, 45 (10): 478-481. |
| [9] | Ibrahim, M. M. and Hussein, R. M. 2006. Variability,heritability and genetic advance in some genotype of roselle (Hibiscus sabdariffa). World Journal of Agricultural Sciences, 2 (3): 340-345. |
| [10] | Wong, L. S. L. and Baker, R. J. 1986. Selection to maturity in Spring wheat. Crop Science, 26: 1171-1175. |
| [11] | Falconer, D.S. 1980. Introduction to Quantitative Genetics. Pages 322 and 323). 2nd ed. Longman, London |
| [12] | El Naim, A M, Jabereldar, A A, Ahmed, S E, Moayad, M B Z, Ibrahim, K. A (2012). Effect of weeds on calyces yield of in traditional agricultural sector of Sudan. International Journal of Plant Research , 2 (2): 1-5 |
| [13] | Louis, S.J, Kadams, A.M., Simon, S.Y. and. Mohammed, S.G, 2013. Combining Ability in Roselle Cultivars for Agronomic Traits in Yola, Nigeria. Greener Journal of Agricultural Sciences, 3 (2): 145-149 |
| [14] | Gasim, S. M. and Khidir, M. O. 1998. Genetic variability of some characters in Roselle (Hibiscus sabdariffa var.sabdariffa L.). University of Khartoum Journal of Agricultural Sciences, 6 (1): 22-33. |
| [15] | Panse, V.G. 1957. Genetics of quantitative characters in relation to plant breeding. Indian Journal of Genetetics and Plant Preeding, 17 (2): 318-328. |
| [16] | Johnson, H.W.; Robinson, H.F. and Comstock, R.E. 1955. Estimates of genetic and environmental variability in soybean. Agronomy Journal. 47: 314-318. |
| [17] | Allard, R. W. 1960. Principles of Plant Breeding. John Wiely and Sons Inc., New York, USA |
| [18] | Sasmal, B.C. and Chakraborty, K. 1977. Variability in quantitative character of kenaf. Acta Agronomica Hungarica, 26:276-283. |
| [19] | Dutta,A.N., Srivastava, S.K. and Yadav, R.A. 1973. Components of variance for fibre yield in jute-cum-mesta varietal test. Indian Agric., 17:257-261. |
| [20] | Mostofa, M.R., Islam, M.R., Morshed Alam, A.T.M., Mahbub Ali, S.M. and Moll, M.A.F. 2002. Gentic Variability, Heritability and Correlation studies in Kenaf (Hibiscus cannabinus L.). Online Journal of Biological Sciences, 2(6):422-424 |
| [21] | Reddy, K.R., Singh, K.P. and Rai,A.K. 1985. Variability and association analysis in okra. Mdras Agric. J., 72:478-480. |
| [22] | Vijay, O.P. and M.S. Manohar, 1990. Studies on genetic variability, correlation and path analysis in okra(Abemoschus esculentus L. (Moench). Indian J. Hort. 47:97-103 |

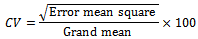 Phenotypic (σ²ph) and genotypic (σ²g) variances were estimated using individual analysis of variance (Tables 1 and 2) as follows:
Phenotypic (σ²ph) and genotypic (σ²g) variances were estimated using individual analysis of variance (Tables 1 and 2) as follows: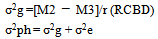 Genetic coefficient of variation (GCV %) was computed following Burton and De Vane[7] as:
Genetic coefficient of variation (GCV %) was computed following Burton and De Vane[7] as: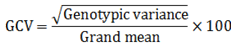 Heritability (h²) in broad sense was estimated for each character using the following procedure:
Heritability (h²) in broad sense was estimated for each character using the following procedure: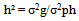 Expected genetic advance under selection (GA) was estimated following Allard[17] Then, the estimated GA was expressed as percentage of the overall mean of the character:
Expected genetic advance under selection (GA) was estimated following Allard[17] Then, the estimated GA was expressed as percentage of the overall mean of the character: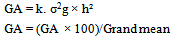 Where:k = the selection differential, and it equals 2.06 for 5% selection intensity
Where:k = the selection differential, and it equals 2.06 for 5% selection intensity Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML





