-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2013; 3(6): 240-243
doi:10.5923/j.ijaf.20130306.06
Organic Carbon in Soil and Biomass of an Alnus nepalensis Forest in Kathmandu, Nepal
Khila Nath Dahal1, Gandhiv Kafle2
1Institute of Forestry, Hetauda, Nepal
2Faculty of Forestry, Agriculture and Forestry University, Hetauda, Nepal
Correspondence to: Gandhiv Kafle, Faculty of Forestry, Agriculture and Forestry University, Hetauda, Nepal.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
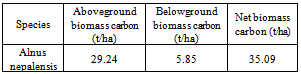
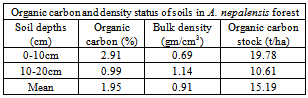
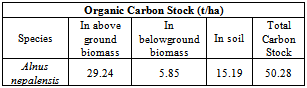
This article reports the results of measuring organic carbon contents in biomass and soil of an Alnus nepalensis forest in Kathmandu. The aboveground and belowground biomass of A. nepalensis were 62.21 t/ha and 12.44 t/ha respectively in the forest. The aboveground and belowground carbon stock in the forest were 29.24 t/ha and 5.85 t/ha respectively. Average organic carbon stock in soil depths 0-10 cm and 10-20 cm were found 19.78 t/ha and 10.61 t/ha respectively. The mean organic soil carbon stock in 0-20 cm soil depth was found 15.20 t/ha and in living biomass was found 35.09 t/ha. The organic carbon stock in 0-10cm soil depth was found 1.86 times more than in soil depth of 10-20 cm. Carbon stored in living vegetation biomass is 2.31 times more than soil organic carbon in the forest upto 20cm soil depth. Soil bulk density in 0-10 cm and 10-20cm soil depths were found 0.69 g/cm3 and 1.14 g/cm3 respectively.
Keywords: Carbon, Soil, Biomass, Alnus nepalensis
Cite this paper: Khila Nath Dahal, Gandhiv Kafle, Organic Carbon in Soil and Biomass of an Alnus nepalensis Forest in Kathmandu, Nepal, International Journal of Agriculture and Forestry, Vol. 3 No. 6, 2013, pp. 240-243. doi: 10.5923/j.ijaf.20130306.06.
Article Outline
1. Introduction
- The exchange of carbon between the atmosphere and terrestrial ecosystems (soil and vegetation) is critical to the patterns of carbon dioxide concentration in the atmosphere [1][2][3][4]. The carbon pool in a terrestrial ecosystem can be broadly categorized into biotic (vegetative carbon) and pedologic (soil carbon) components. Forests are the largest carbon stock in terrestrial ecosystems[5][6][7][8][9] and estimated to be about 1150 Gg, of which 49% is in boreal forests, 37% in tropical forests and 14% in temperate forests[10]. Soils are viable sinks of atmospheric carbon (C) and may significantly contribute to mitigation of global climate change[11][12] [13][14]. Soil organic carbon (SOC) stocks in forest soils fluctuate from 50 to more than 200 Mg/ha, depending upon the climate and soil conditions, the age and type of the tree stand, and management practices[15].A study by FAO[16] showed that 496 million metric tons of organic carbon is stored in soils at forest and shrub land of Nepal. Bajracharya et al.[17] estimated soil organic carbon storage in Nepal's middle hills to be around 423.7 mt C. By depth, forest and shrub land have higher amount of soil organic carbon (2.0% and 2.3% respectively) than the cultivated soils in the top layer between 1 and 30 cm.Vertical patterns of Soil Organic Carbon (SOC) can contribute as an input or as an independent validation for biogeochemical models and thus provide valuable information for examining the responses of terrestrial ecosystems to global change[18][19][20]. Thus, improved knowledge of distribution of SOC across different soil depth is essential to determine whether carbon in deep soil layers will react to global change and accelerate the increase in atmospheric carbon dioxide (CO2) concentration [21]. In this context, this research was carried out to quantify levels of organic carbon in soil at two depths and biomass of an Alnus nepalensis Forest in Kathmandu, Nepal.
2. Materials and Methods
- The study was conducted in Rajat Uddhyan Community Forest, located in Mulpani-6, in Kathmandu District of Nepal. It is about 3 km away from Chabahil Chowk. The forest is a community managed forest with 1.5 hectares area. Alnus nepalensis is dominant species with more than 90 percent coverage. The A. nepalensis in the forest are only of pole sizes.Vegetation MeasurementThe area of forest was 1.5 hectares, so all the poles of A. nepalensis were measured for diameter and height. Diameter at breast height (Dbh) was measured using diameter tape. The distance between the A. nepalensis pole and measurer was measured using Linear Tape. Ground slope, and top and bottom angle to the pole of A. nepalensis was measured using Clinometer. The information thus taken was used to calculate the height of the measured pole of A. nepalensis. Soil MeasurementTen pits of 40cm depth were dug for soil sample collection in the study area. For the purpose of estimating bulk density, three individual soil samples of approximately 200 cm3, one each from two depths (0-10 cm, and 10-20 cm) were collected with the help of a standardized 200 cm3 metal soil sampling corers. Similarly for the purpose of analysing organic carbon, three individual soil samples of approximately 100 gram, one each from two depths (0-10 cm, and 10-20 cm) were collected.Soil samples were placed in sample bags, labelled and transported to the laboratory for further analysis. The overall field measurement methods were guided by ANSAB[22]. Vegetation Data AnalysisThe following biomass model was used to estimate aboveground pole biomass as per guidelines given in[23] on the basis of climate and forest stand type.AGPB = 0.0509 *ρD2HWhere, AGPB = above ground biomass of pole (kg),D = Diameter at Breast Height (cm), ρ = Wood Specfic Gravity (g/cm3)H = Height of tree (m).After taking the sum of all the individual weights (in kg) and dividing it by the forest area, the biomass stock density was attained in kg m-2. This value was converted to t ha-1 by multiplying it by 10. The biomass stock density was converted to carbon stock density after multiplication with the IPCC[24] default carbon fraction of 0.47. Belowground pole biomass was estimated as 20% of aboveground pole biomass using MacDicken[25] root-to-shoot ratio value of 1:5.Soil Data AnalysisSoil bulk density was determined using the soil core samples[26]. The soil organic carbon (SOC) concentration was determined by dry combustion of oven-dry soil samples[27].Bulk Density of Soil = (Oven dry weight of soil in gram) / (Volume of the soil in cm3) Soil Organic Carbon (ton per hectare) = Organic carbon content% × soil bulk density (gram/cm3) × soil layer depth (cm).
3. Results and Discussion
- Biomass of Alnus nepalensisThe aboveground biomass and belowground biomass of A. nepalensis forest was found to be 62.21 t/ha and 12.44 t/ha respectively. Mean living biomass of A. nepalensis was found 74.65 t/ha in the forest (table 1).
|
|
|
|
4. Conclusions
- A comparison of the organic carbon stock values in soils of A. nepalensis forest show that the carbon stock tonnes per hectare decrease with soil depth upto 20cm from the ground level. The organic carbon content in living biomass of A. nepalensis was found around double than the total soil organic carbon content upto 20cm depth. Total organic carbon stock in the A. nepalensis forest was found 50.28 t/ha. Further studies on soil organic carbon in deep soil layers and A. nepalensis forest of varying age class are highly recommended for generalization of the findings.
ACKNOWLEDGEMENTS
- The authors are highly grateful to the ComForM project of Institute of Forestry for supporting this research. We got sincere support from the user group committee members of the Rajat Uddhyan Community Forest during field work and are highly grateful to their involvement.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML