-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2013; 3(5): 191-197
doi:10.5923/j.ijaf.20130305.01
Managing Soybean for Enhanced Food Production and Soil Bio-Fertility in Smallholder Systems through Maximized Fertilizer Use Efficiency
Ezekiel Mugendi Njeru1, 2, John Muthini Maingi1, Richard Cheruiyot1, Gitonga Nkanata Mburugu3
1Department of Plant and Microbial Sciences, Kenyatta University, P.O. Box, 43844-00100, Nairobi, Kenya
2Institute of Life Sciences, Sant’ Anna School of Advanced Studies Piazza Martiri della Libertà n.33 – 56127, Pisa, Italy
3Meru University of Science and Technology, P.O. Box, 972-60200, Meru, Kenya
Correspondence to: Ezekiel Mugendi Njeru, Department of Plant and Microbial Sciences, Kenyatta University, P.O. Box, 43844-00100, Nairobi, Kenya.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The production of promiscuous soybean by smallholder farmers in Kenya would improve soil fertility through biological nitrogen fixation (BNF), boost food security, and contribute to generation of cash. The present study was conducted to determine the effects of soil amendments on growth and yields of promiscuous soybean cultivars under varying soil carbon levels. Field experiments using early maturing SB 19 and late maturing SB 20 promiscuous soybean cultivars and different levels of phosphorus (P), potassium (K) and sulphur (S) fertilizers were conducted in two sites in the south-eastern slopes of Mt. Kenya, approximately 1500 m above sea level. The soybean cultivars were observed for phenology, plant biomass production, pod fresh weight, 1000 seed weight and haulm weight. Significant differences were observed on most yield components due to field carbon level, soybean cultivar and fertilizer amendments, while the plant height was only affected by fertilizer application and soy bean cultivar. However, the effects due to the interaction of these factors were not significant. Therefore, the benefits of combined use of BNF by soybean and application of PKS fertilizers could be a promising entry point into maximized fertilizer use efficiency by smallholder systems in Kenya.
Keywords: Promiscuous Soybean, Soil Amendments, Biological Nitrogen Fixation, Smallholder Farmers
Cite this paper: Ezekiel Mugendi Njeru, John Muthini Maingi, Richard Cheruiyot, Gitonga Nkanata Mburugu, Managing Soybean for Enhanced Food Production and Soil Bio-Fertility in Smallholder Systems through Maximized Fertilizer Use Efficiency, International Journal of Agriculture and Forestry, Vol. 3 No. 5, 2013, pp. 191-197. doi: 10.5923/j.ijaf.20130305.01.
Article Outline
1. Introduction
- Soybean (Glycine max (L.) Merril) is an important source of high quality, inexpensive protein and oil, presently cultivated worldwide under varying climatic conditions[1]. Compared to other foods that are rich in proteins such as animal meat, fish, eggs, and milk, soybean is by far the cheapest source of protein for smallholders in Africa. The amount of soybean protein consumed by humans worldwide is currently low, although there is increasing public and commercial interest since the crop could be a major source of dietary protein for the future. Another advantage of soybean is that it improves soil fertility by fixing atmospheric nitrogen through biological nitrogen fixation (BNF)[2-3]. This would be a major benefit to smallholder farming systems in Kenya where soil degradation and nutrient depletion have gradually increased and now pose serious threats to sustainable food production.There has been a slow growth in production of soybeans by smallholders in Kenya over the years. Although soybean forms a major alternative source of proteins and cooking oil, local production in Kenya is still low at 4335 tons in year 2011[4]. The main reasons for slow growth are postulated to be lack of awareness about soybean management (production, processing, and use), low yields and few markets[5]. Soybean production in Kenya can further be improved by increasing the output from the land presently under cultivation and expanding production to the land yet unexploited. Most parts of Western, Central and Eastern Kenya regions receive adequate rainfall, have well drained soils of moderate to high fertility with a pH range of 5.5–7.5, and are hence considered to have a high potential for soybean production and future expansion[6].Similar to many parts of Kenya, crop yields in Meru South are presently low mainly due to declined soil fertility status mainly attributed to continuous cropping without addition of fertilizers or manure[7-8], nutrient loss through soil erosion and leaching. Moreover, the cropping sequences are poorly planned thus do not maximize on agroecological soil nutrient cycles. Being resource poor, most smallholder farmers in Meru South like their counterparts in the rest of sub-Saharan Africa (SSA) typically apply negligible amounts of mineral fertilizers. Despite repeated demonstrations of the usefulness of green manures in enhancing soil fertility in Meru South[8] the practice of using green manure is still limited. Proven district-level recommendations of fertilizer rates based on several years’ field investigation are now in place [9]. However, they rely on blanket application approaches that are often not readily adoptable by smallholder farmers because they are based on optimizing yield, rather than maximizing the efficient use of scarce inputs. It is therefore essential to develop appropriate technologies to improve and manage soil fertility based on judicious use of organic and inorganic fertilizers while at the same time taking measures that will enhance preservation of the land resource base by smallholder farmers.Soybean farming is one of the most cost-effective ways in which smallholder farmers can maintain soil fertility and yet reap other benefits from the crop. They are among legumes known for their high nitrogen fixing ability[10], thus improving soil nitrogen (N) content[11]. Promiscuous soybean genotypes nodulate and fix nitrogen effectively with diverse indigenous rhizobia available in the soil while non-promiscuous genotypes form symbioses with specific rhizobial strains thus limiting their N- fixing ability[12]. Recent studies on promiscuous soybeans cultivars in the study area have reported increased nodulation upon inoculation and fertilizer application[13-14], which could increase BNF benefits in smallholder systems. Therefore, although soybean is relatively a new crop to many smallholders in Kenya, its cultivation is expected to gain popularity in the near future because of the increasing demand for food, fodder and search for alternative soil fertility management strategies which rely more on agroecological cycles.Nodulation and nitrogen fixation in legumes occurs effectively if other mineral elements such as Phosphorus (P), Potassium (K) and Sulphur (S) are present in the soil[15]. This leads to increased yield components in these legumes. Therefore, this necessitates constant addition of PKS fertilizers to boost the soil mineral nutrient level especially in low carbon sites. Much research on the effect of soybean inoculation and P application has been conducted[16]. However, there is limited information on the effects of soil amendments with PKS fertilizers on production of promiscuous soybean cultivars in smallholder systems. The main objective of this study was to investigate the effects of soil amendments with PKS fertilizers on growth patterns and yield components of two promiscuous soybeans cultivars in low and high carbon level sites.
2. Materials and Methods
2.1. Study Area
- Field experiments were conducted in two sites located in Tharaka Nithi County (Meru South) in the south-eastern slopes of Mt. Kenya, approximately 1500 m above sea level. The area is in upper midlands 2 and 3[17], with soils that are mainly humic nitisols[18] derived from basic volcanic rocks. The soils are deep, well weathered with moderate to high inherent fertility. The average maximum temperature is 27℃; the minimum is 14℃ while the average temperature is 20.5℃. The area receives annual rainfall varying from 500 to 2200 mm, bimodally. Long rains occur between March and June while short rains fall from October to December. Eight farms were identified in each area and the cropping history noted. In each farm, two sites were selected based on total organic carbon (C) (one site with the lowest C and one site with the highest C). A mixture of concentrated sulphuric acid and aqueous potassium dichromate was used to determine the soil organic carbon[19].
2.2. Field Preparation and Experimental Layout
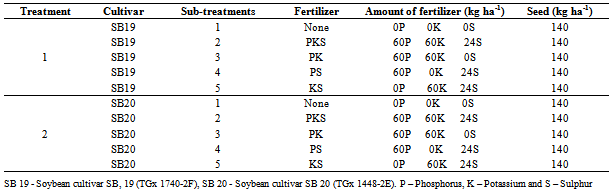
- The experiment was laid out as a randomized complete block design (RCBD) with each farm serving as a replicate. The promiscuous soybean cultivars SB 19 (TGx 1740-2F), an early maturing cultivar and SB 20 (TGx 1448-2E), a late maturing cultivar were the main treatments while fertilizer inputs were the sub-treatments. Plot sizes measured 4 m by 2.7 m. A total of two treatments and five sub-treatments were used (Table 1). The experimental fields were cleared of grasses and other prevalent weeds using mechanical methods, after which they were demarcated. The fields were ridged at 45 cm intervals to a depth of 30 cm. Soybean seeds of high viability and quality were carefully selected to increase chances of uniform germination. All the fertilizer inputs were applied in the planting line on the trough and incorporated before planting. After the application of fertilizers, soybeans seeds were planted in rows, at a spacing of 45 between rows and cm 5 cm within rows. Weed control was done manually by periodically scouting the plots and uprooting the weeds wherever necessary.
|
2.3. Phenological Measurements and Shoot Biomass
- The phenological changes of the soybean crops were regularly determined. These changes included percentage emergence at 3 weeks after planting (WAP), plant height at 10 WAP, flowering, peak biomass (podding), and physiological maturity. At 50% podding, one 50 cm long row of plants (10 plants with a space of 5 cm between each plant) was selected leaving 50 cm from the plot borders. The plants were cut using a sharp knife at about 5 cm above the soil level. Immediately after cutting the above ground biomass was put in labeled polythene bags and then allowed to air dry. Shoot biomass of the plants was determined after oven drying at 65℃ to constant weight.
2.4. Yield Components
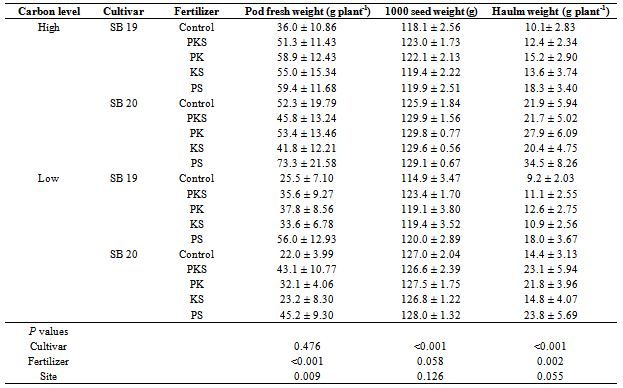
- Physiological maturity was considered to have taken place when 95% of the plants had turned golden yellow[20] and 75% of the plants had their pods filled with seeds and hardened[21]. At harvest, pods were separated from haulms, weighed and then sun dried until they were ready for threshing. After the grain was threshed, the pod walls were combined with the haulms to comprise the crop residue. The threshed seeds were oven-dried to a moisture content of about 8% and the weight determined.
2.5. Data Analysis
- Data collected on crop emergence, plant height, plant biomass production, pod fresh weight per plant, a thousand grain weight and haulm weight were analyzed using ANOVA procedure in SAS (Statistical Analysis System) software[22]. Data on crop emergence and yield components were arcsine or log(x+1) transformed to fulfill the assumptions of ANOVA. The reported data in tables were back-transformed. When ANOVA indicated statistical significance of a treatment effect (P<0.05), the means were separated using Tukey`s HSD test.
3. Results and Discussion
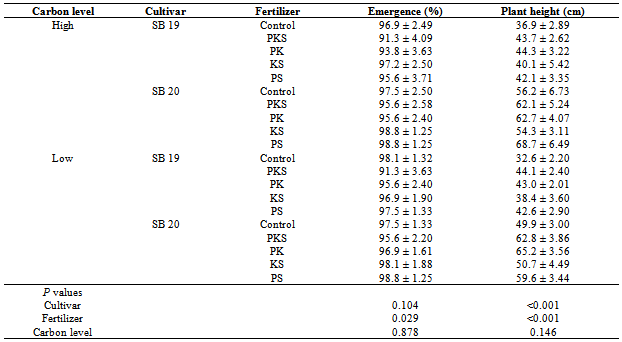
- In this study, the response of two soybean cultivars to different fertilizer combinations under high and low carbon level sites was studied. The experiment was conducted in smallholder farms and in collaboration with farmers in the study area allowing their contribution at each experimental phase. Our results demonstrate significant effects on some important soybean parameters based on soybean cultivar, field carbon level and fertilizer treatment. Crop emergence was significantly (F=2.8, P=0.029) lower after the application of PKS fertilizer compared to the other fertilizer treatments (Table 2). However, the emergence was not significantly affected by the soybean cultivar, soil carbon level and their interactions. Some of the seedlings that germinated dried up due to lack of rainfall for about two weeks after planting. After germination the early maturing cultivar SB 19 took 60 days to 50% attain flowering, 81 days to form 50% of the pods and 128 days to mature. Cultivar SB 20 took 70 days to attain 50% flowering and 90 days to form 50% of pods and 148 days to mature (Table 3).Application of PKS, PK, and PS fertilizers significantly (F= 6.6, P<0.001) influenced the plant height compared to KS and the Control treatments. Plant height was also significantly (F= 109.0, P<0.001) affected by soybean cultivar whereby, cultivar SB 20 had a higher mean height (59.2±1.5 cm) compared to SB 19 (40.8 ± 1.0 cm). The greatest plant mean height (68.7 ± 6.5 cm) was observed in cultivar SB 20 grown after application of PS fertilizer on high carbon fields (Table 2). There were no significant interactions between the treatments.
|
|
 | Figure 1. Cultivar SB 20 grown on a high carbon field amended with 60 kg P ha-1 60 kg K ha-1 and 24 kg S ha-1 fertilizers |
 | Figure 2. Cultivar SB 20 grown on a low carbon level field with no fertilizer inputs (Control) |
|
4. Conclusions
- The results from this study clearly demonstrate:- (i) The importance of P in the fertilizer combinations used for maximized soybean production by smallholders in the study area (ii) Better production of the late maturing cultivar SB 20 in the study area (iii) The strong influence of soil carbon level on soybean production within the same farm, although farmers in the study area own relatively small pieces of land and thus the distance between the high carbon and low carbon field was quite small. With the majority of the population in the study area being dependent on the limited agricultural land (requiring no irrigation), improved soybean production by modest application of PKS fertilizers will boost food security and soil fertility in the study area. This will increase crop yields in Kenya and therefore, improve the livelihood of rural populations by offering good and reliable economic returns. Among the most immediate viable strategies as proposed by this study is the amendment of these soils with the currently underutilized organic and inorganic fertilizers and the use of biological nitrogen fixation by legumes. Besides this, new strategies must be defined to connect more closely applied research with the needs of smallholder farmers.
ACKNOWLEDGEMENTS
- The authors are grateful to Kenyatta University- Department of Plant and Microbial Sciences for providing the necessary laboratory facilities and acknowledge the cooperation and contributions by Meru South farmers.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML