-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2013; 3(4): 159-161
doi:10.5923/j.ijaf.20130304.06
Characterization of Phytophthora nicotianae Pathogenic to Chamaerops humilis in Iran
Eisa Nazerian1, Mansureh Mirabolfathi2
1National Research Station of Ornamental Plants, Mahallat, Iran
2Plant Protection Research Institute, Tehran, Iran
Correspondence to: Eisa Nazerian, National Research Station of Ornamental Plants, Mahallat, Iran.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Phytophthoranicotianaewas isolated and identified using standard taxonomic criteria from Chamaeropshumilisvar. argenteashowing inner basal leaves rot in Markazi province of Iran. Disease symptoms appeared as discoloration, water soaking on interior basal leaves. Collapse of an affected plant occurs less than one month. Lesion expands from the inner basal leaf to the tip. All affected plants turned to pale gray or silvery in advanced stage of disease. In pathogenicity test with insertion of mycelial plug of respective isolates, similar symptoms produced as naturally infection. This is the first report of P. nicotianaein C.humilis from Iran.
Keywords: Blue Mediterranean Fan Palm, Landscape Plant, Ornamental Plants, Disease
Cite this paper: Eisa Nazerian, Mansureh Mirabolfathi, Characterization of Phytophthora nicotianae Pathogenic to Chamaerops humilis in Iran, International Journal of Agriculture and Forestry, Vol. 3 No. 4, 2013, pp. 159-161. doi: 10.5923/j.ijaf.20130304.06.
1. Introduction
- Ornamental plants are one of the major economic plant commodities in Iran, grown either in greenhouse or in the field; however the majority of ornamental plants are cultivated in northern and central of the country. Recently, many ornamental plants varieties were introduced and cultivated across the country, subsequently; new disease occurred and cause loss to growers. Susceptibility of many ornamental plants to Phytophthora nicotianae was reported previously[11-8]. Phytophthora nicotianae strains are able to infect different hosts. To date more than 301 different hosts of this pathogen including Allium cepa, Dianthus caryophillus, Lycopersicum esculentum and Euphorbia pulcherrima were reported[4-17]. Phytopathogen Phytophthora nicotianae have been first reported on Chamaerops humilis var. argentea from Italy in 2011[5]. Moist environmental condition and unsuitable irrigation methods led to spread propaguls (sporangia and zoospore) of P. nicotianae. The disease seems to be spread quickly and there is cause for concern if diseased plants are found among healthy. Field observations of disease spread and past cultural histories of different plantings provide evidence that the disease may be rapidly spread by cultural practices and overhead irrigation water. Disease development can be reduced by sanitation practices include, the removal of plant debris, sterilizing pot and use of disease free plant material. Many growers also treat plants with anti-oomycetes fungicides such as phenylamide, mefenoxam and metalaxyl [17]. This study report symptomatology and pathogenicity of the causal agent on C. humilis.
2. Material and Methods
- In the summer of 2012, 15% of a nursery stock, approximately to 4000 potted blue Mediterranean fan palms growing in an ornamental nursery in Markazi province showed dieback. Diseased tissues showing advanced disease symptoms were collected and disinfected with 5% NaClO for 3 min. Small pieces (0.5-1 cm) were placed onto corn meal agar (CMA) and potato dextrose agar (PDA) media. For better production of sporangia, mycelia tips were placed onto V-8juice agar supplemented with 200ppm ampicillin, 50ppm mycostatin and 10ppm pentachloronitrobenzen[9]. Seven pure cultures of fungi were obtained using mycelia tips culture after incubation at 25°C for 7 days[10]. The width and length of 12 sporangia were measured for each isolate. The growth speed at 36°C was measured during 5 days, on 3 replicates for each isolate, on V-8 agar medium in the dark. Oospore production and determination of mating types were done using V8 medium. All isolates were stored in test tube on 5% V-8 juice agar at 25°C.In pathogenicity test C. humilis were grown under greenhouse condition in plastic pots (30 x30 cm) at 25°C. The soil mixture was as grower used and sterilized prior to use at 120°C for 30min.For inoculum preparation, all isolates were grown on V-8 juice agar at 25°C for 5 days. To stimulate sporangia formation, pieces of each inoculum derived from V-8 juice agar was cultured in to 100 ml of sterile 1% potassium nitrate solution in five petridishes[7]. These cultures were grown under uv light at 25°C for 5 days. Pathogenicity tests were performed by wound-inoculation with a cork borer of 8 three-year-old potted fan palms. After disinfection of the inoculation surface with 70% ethyl alcohol, a mycelial plug of seven-day-old colonies grown on V-8 juice agar was inserted into the basal stem and the hole was covered with the removed tissue and sealed with Parafilm®[5].To compare the isolates behavior in pathogenicity and aggressiveness, an experiment was conducted in a completely randomized design with 3 replications per isolate and 8 plants per replication. Disease severity readings were made at 10 and 20 days after inoculation on each plant of every pot[15].The experiment was carried out at the National Research Station of Ornamental Plants in Iran during 2012. Pots were irrigated daily with tap water. The greenhouse condition was conducted at 25 ± 2°C and 43% RH.Classification to species was confirmed by ribosomal DNA sequencing. After DNA extraction from pure culture, amplification of DNA in PCR was carried out using ITS4 and ITS6 primers[16]. The PCR consisted of 1 cycle of 95°C for 2 min; 30 cycles of 95°C for 20 s, 55°C for 25 s, 72°C for 50 s; and a final cycle of 72°C for 10 min[3]. A 853bp band was obtained from amplification of each ITS-PCR product in 1.5 % agarose gel w/v run in TG buffer (3gr/li Tris-Base MW = 121.10, 28.8 gr/li glycine MW = 75.07), stained with 1.0 % ethidium bromide, visualized under uv light and photographed.
3. Results and Discussion
- A dark brown rot on the petiole base and blight of the unopened spear leaves were the main disease symptoms (Figure1).
 | Figure 1. Natural infection of blue Mediterranean fan palm caused by Phytophthora nicotianae, (a: unopened spear leaves, b: browning of basal stem) |
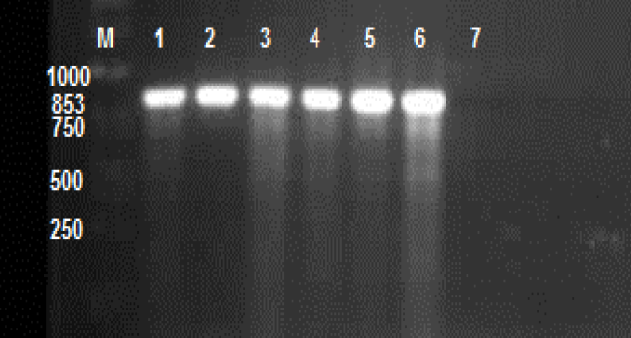 | Figure 2. ITS-PCR banding pattern of six isolates (lane 1-6) of P. nicotianae isolated from blue Mediterranean fan palm. M; Molecular marker 1 kb, lane 7; Control without DNA |
 | Figure 3. Cross section of bud basal rot caused by Phytophthora nicotianae in pathogenicity test, photographed after 10 days |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML