-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2013; 3(3): 94-97
doi:10.5923/j.ijaf.20130303.04
Improving the Rooting Capacity of Stem Cuttings of Schefflera arboricola, Ficus benjamina and Syringa amurensis by Influenced different Concentrations of Indole-3-Butyric Acid (IBA)
Karimiyan M. A.1, Dahmardeh M.2, Khamari I.2
1M.S of Agriculture, Department of Agronomy, Faculty of Agriculture, University of Zabol
2Asistant professor of Agronomy, Department of Agronomy, Faculty of Agriculture, University of Zabol
Correspondence to: Dahmardeh M., Asistant professor of Agronomy, Department of Agronomy, Faculty of Agriculture, University of Zabol.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The experiment was carried out to investigate the effect of different concentration of Indole-3-butyric acid (IBA) on the rooting ability of Scheffleraarboricola, Ficusbenjamina and Syringaamurensis. The treatments were factor A (IBA concentration) include four levels included: control (without IBA), 2000, 4000 and 6000 ppm IBA and factor B (three varieties) included: Scheffleraarboricola, Ficusbenjamina and Syringaamurensis. Treatment was evaluated in a factorial experiment at completely randomized design (CRD) with 3 replications. According to the obtained results, there was significant difference between IBA treatment and control on No. of roots per rooted cutting, Root length per rooted cutting, Root wet wt. per rooted cutting and Root dry wt. per rooted cutting. The best effect of different levels of IBA on the number of root (6.62 roots per plant) was obtained at 6000 ppm IBA. The highest of no. of roots per rooted cutting, root length per rooted cutting, root wet wt. per rooted cutting and root dry wt. per rooted cutting in the between of varieties was obtained in Ficusbenjamina. It can be concluded that using IBA affected rooting ability in all parameters in three plants.
Keywords: Auxin, Root Length, Number of Root
Cite this paper: Karimiyan M. A., Dahmardeh M., Khamari I., Improving the Rooting Capacity of Stem Cuttings of Schefflera arboricola, Ficus benjamina and Syringa amurensis by Influenced different Concentrations of Indole-3-Butyric Acid (IBA), International Journal of Agriculture and Forestry, Vol. 3 No. 3, 2013, pp. 94-97. doi: 10.5923/j.ijaf.20130303.04.
Article Outline
1. Introduction
- Scheffleraarboricola is a flowering plant in the family Araliaceae native to Taiwan. It also goes by the common name "Dwarf Umbrella Tree." It is an evergreen shrub growing to 3-6 m tall, free-standing, or clinging to the trunks of other trees. The leaves with 7-9 leaflets, the leaflets 9-20 cm long and 4-10 cm broad (though often smaller in cultivation). Ficusbenjamina, commonly known as the weeping fig, is a member of Moraceae family. This plant is increased through vegetative method, cutting[16]. Khoshkhoy (1991) to express that cutting is an easiest and cheapest manner to mass propagation and production plants more uniform and genetically similar to the parent. Tree lilacs (Syringaamurensis) similarity small trees, and can reach a height of 30 feet in early summer; tree lilacs produce spectacular clusters of off-white, privet-like blooms. A common cultivar is the Japanese tree lilac (Syringaamurensis japonica), which produces great clusters of yellow-white flowers late in the season. It grows 25 to 30 feet tall. Syringa L. the lilacs, is well known for beautiful and fragrant flowers, and its species have been widely cultivated as ornamentals[10]. Twenty-two to 28 species of Syringa have been recognized, and are issued in southern Europe and East Asia[4, 8, 14]. First adventurous roots appear from callus and they are main roots for cuttings. Callus includes high amount of auxines[11, 12]. Softwood finals of easy-to-root varieties a rooting hormone. With more mature wood, a rooting hormone such as IBA (3-indolebutryic acid) at 2000-6000 ppm is commonly used[13]. Gilman (1999) showed that higher concentrations may be needed with more difficult-to-root cultivars[9]. Wiesman et al (1988) were shown that one of the most important uses of synthetic plant growth regulators in horticulture is the use of auxin for the comparable of root formation in cuttings. The auxin indole-3-acetic acid (IAA) was the first plant hormone to be used in rooting in 1935[19]. Epstein and muller (1993) showed that several new synthetic auxins including indole-3- butyric acid (IBA) and naphthalene acetic acid (NAA) improvement rooting. The promoting effect of IBA on rooting is basically the result of its replacement to IAA in plant tissue[7]. However, IAA which is needed for the rooting process, whereas free IAA released from IBA is not oxidized by peroxidase and remains at the base of the cutting[8]. Barzegar et al. (2004) reported that a proper rooting media is an important step in horticultural and ornamental plants increasing[3]. BabashpourAsl et al. (2012) shown on Bougainvillea sp. were shown that significant diversity between IBA treatment and control on the number of true roots per cutting[1]. Moalemi and Chehrazi (2005) were shown that high percentage of rooting was for leafy cutting. In that study, the highest number of roots was obtained in leafless cutting (9.23 roots per plant) at 2000 mg L-1 IBA. The best effect of different levels of auxin on root length (5.79 cm) was at 1000 mg L-1 NAA. In control treatment, without auxins, the cuttings rooted 22.5-93% but their root system was weakly developed[15]. Czekalski (1989) showed that rooting medium has not been said, but in the latter one, rooting medium was sand[5]. Affirmative correlation between IAA amount and the number of adventurous roots was found in Chrysanthemum morifoliumcuttings[18]. BarzgarTorghabeh (2003) in Ginkgo biloba L. were results that IBA at 4000 ppm, increased percent of rooting in stem cuttings, but higher concentration decreased it. The best effect of different levels of IBA on the number of basic roots per cutting (8.67 roots per cutting) was obtained at 2000 ppm IBA[2]. So the prepared research project was researched to find out the optimum Auxin (IBA) hormone concentration on Scheffleraarboricola, Ficusbenjamina andSyringaamurensis rooting of hardwood cuttings under green house conditions.
2. Materials and Methods
- This study was carried out at Department of Agriculture, Zabol University, Iran in 2012. The treatments were control (without IBA), 2000, 4000 and 6000 ppm IBA. The cuttings were treated with IBA solution for 5 seconds and directly transferred to the rooting. Sands wind was used as a rooting substrate. Treatment was evaluated in a factorial experiment at completely randomized design (CRD) with 3 replications. Each replication consisted of 10 cuttings. The formulations were ready by dissolving the net compound in NaOH and water. Cutting prepared after discarding the semi-hardened upper parts bearing 3-4 buds. The basal end of cuttings were floating briefly in a fungicide solution (2/1000 v/v Zineb) treatment with IBA solutions. During the rooting period, the mean temperature was entered as 20℃ and the mean moisture was registered at 45 -50% in the greenhouse.
2.1. Preparation of the Cuttings
- Stem of Scheffleraarboricola, Ficusbenjamina and Syringaamurensiscuttings with about 5-10 mm in diameter, horizontally in small pieces (15 - 20 cm long with 3 - 4 buds/cutting), cutting at the base 2 cm below a node and tendrils in the rooting hormone. A stock solution was prepared by winding up in 0.1 N (NaOH), after that watery (0, 2000, 4000 and 6000 ppm) were prepared. The bases of the cuttings were wet for 5 seconds in the prepared treatments; each treatment was replicated three times with 10 cuttings per replicate.
2.2. Rooting Conditions
- The experiment was conducted in a greenhouse at Zabol University, research center of Agriculture. Average air temperature was 20 - 25°C and average relative humidity was 40-45%. Cuttings were watered once every 2 - 3 days up to 60 days, then observations on rooting response were recorded after the 60 days of supplement.
2.3. Parameters Measured
- The following measurements and readings were taken after 60 days from supplement (planting) date.
2.4. Average Number of Roots per Rooted Cutting
- All produced roots from the rooted cuttings were counted and then the total numbers of roots were divided by the total number of rooted cuttings.
2.5. Average Root Length per Rooted Cutting
- All produced roots were removed, their lengths were measured and the total of the roots length was divided by the total number of rooted cuttings.
2.6. Average Root Wet Weight per Rooted Cutting
- Freshly harvested roots per replicate and weight were taken by digital scale balance and medium readings were considered per rooted cutting.
2.7. Average Root Dry Weight per Rooted Cutting
- Freshly harvested roots per replicate were dried in an oven at 74°C for 24 hours to a constant weight and then placed in desiccators until their temperature dropped. Weight was taken by digital scale balance and average readings were considered per rooted cutting.
2.8. Experimental Design and Statistical Analysis
- treatments were conducted in factorial experiment at a completely randomized design (CRD) with tree replicates. All data obtained were statistically analyzed according to the design used in this experiment as outlined by Steel and Torrie (1980) differences between treatment means were compared by using Least Significant Difference at 5 % significant level[17].
3. Results and Discussion
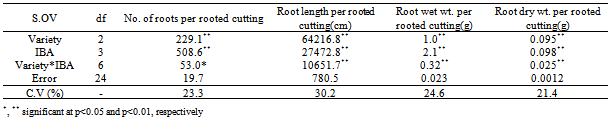
- Analysis of Variance and alterations of the independent and dependent variables for all rooting traits are presented in Table 1. significant differences were recognized for root parameters.
3.1. Average Number of Roots per Rooted Cutting
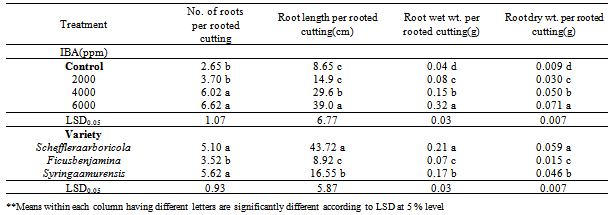
- Significant differences were observed between all the used IBA concentration treatments (Table 1); cuttings treated with 6000 ppm IBA produced the highest number of roots (6.62) per rooted cutting, while control treated cuttings produced the lowest number of roots (2.65) per rooted cutting without being significantly different from 2000 ppm IBA treatment. These results suggest that any IBA concentration can be used, but its better use the highest concentrations. Interaction between variety and IBA concentration was showed that the highest of No. root per rooted cutting was obtained at Scheffleraaraboricolato 6000 ppm IBA (8) that no significant different with Syringaamurensis at 6000 ppm IBA (7.32).
3.2. Average Root Length per Rooted Cutting
- The longest roots (39.0 cm) were obtained by 6000 ppm IBA treatment (Table 2), that statistical differences with the 4000 ppm IBA treatment, while the shortest roots (8.65 cm) were obtained by the control treatment, without statistical different with the 2000 ppm IBA treatment.
|
|
|
3.3. Average Root wet Weight per Rooted Cutting
- Cuttings treated with 6000 ppm IBA gave the highest average root wet weight (0.32 g) per rooted cutting (Table 2), which was significantly higher than the control, 2000 and 4000 ppm IBA treatments. Control treatment gave the lowest average wet weight (0.04 g) per rooted cutting with being significantly different from 2000 and 4000 ppm IBA treatments. Interaction between varieties and IBA concentration was showed that the highest of Root wet Weight per Rooted Cutting was obtained at Scheffleraaraboricolato 6000 ppm IBA (0.475 g) that significant different with all treatments.
3.4. Average Root dries Weight per Rooted Cutting
- Cuttings treated with 6000 ppm IBA gave the highest average root wet weight (0.071 g) per rooted cutting (Table 2), which was significantly higher than the control, 2000 and 4000 ppm IBA treatments. Control treatment gave the lowest average wet weight (0.009 g) per rooted cutting with being significantly different from 2000 and 4000 ppm IBA treatments.
4. Conclusions
- Cuttings treated with 6000 ppm (IBA) produced the best results of Number of roots per rooted cutting, Root length per rooted cutting, Root wet weight per rooted cutting and Root dry weight per rooted cutting with being significantly different from cuttings treated with others IBA. The highest number of roots per rooted cutting was obtained by 6000 ppm IBA treatment with being significantly different from all other treatments and the control treatment, which produced the lowest number of roots. The longest roots and the highest average root dry weight per rooted cutting were obtained by 6000 ppm IBA treatment, while the shortest roots and the lowest average root dry weight per rooted cutting were obtained by the control treatment.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML