-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2013; 3(2): 52-59
doi:10.5923/j.ijaf.20130302.04
Fungal Species Associated with Graft Union on Grapevine, its Impact on Graft Failure Process and Attempted Solutions in Egypt
Abo Rehab, M. E. A., A. K. M. Korra, M. A. M. Kamhawy, K. Y. A Youssef
Plant Pathology Research Institute, Agricultural Research Center, 9 Gamaa St., 12619 Giza, Egypt
Correspondence to: K. Y. A Youssef, Plant Pathology Research Institute, Agricultural Research Center, 9 Gamaa St., 12619 Giza, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
This study was performed during 2011-2012 in four different locations in Egypt to investigate the fungal species associated with graft union cause grafting failure process. Phomopsis viticola was the most frequent pathogen isolated from graft failure seedlings in graft union in all localities followed by Botryodiplodia theobromae. Whereas, the other isolated fungi (Phoma sp., Fusarium solani and Alternaria solani) were negligible. Different approaches for disease management including chemical and biological methods were carried out. Among different fungicides, Topsin M 70% (WP) and Kemazed 50% (WP) gave the best results for controlling fungal pathogens cause graft failure, followed by Billis 38% (WG), Saprol 19% (DC), Syllit 40% (SC) and Conazol 10% (EC). As alternative control means, Bio-Zied (Trichoderma album), Rhizo-in (Bacillus subtilis) and Bio-Arc (Bacillus megaterium) reduced the percentage of disease incidence by 40.06 %, 26.92 % and 25.18 %, respectively. Based on the results obtained by this study, phytopathogenic fungi can be considered one of the most important factors influencing graft union success of grapevine in Egypt.
Keywords: Grapevine, Graft Union, Fungal Pathogen, Graft Failure
Cite this paper: Abo Rehab, M. E. A., A. K. M. Korra, M. A. M. Kamhawy, K. Y. A Youssef, Fungal Species Associated with Graft Union on Grapevine, its Impact on Graft Failure Process and Attempted Solutions in Egypt, International Journal of Agriculture and Forestry, Vol. 3 No. 2, 2013, pp. 52-59. doi: 10.5923/j.ijaf.20130302.04.
Article Outline
1. Introduction
- In Egypt, grape is a wildly spread fruit crop and considered to be the second most important fruit crop following citrus fruits. Recently, the total area cultivated with grapes in Egypt has increased covering 64034 hectares with a production estimated to be 1360250 tones[12]. Grape is easily propagated by cutting and grown through a wide range of soils. The major reason to use rootstocks lies to their resistance to some biotic and abiotic problems. The major criteria for rootstocks choice in the order of their importance are phylloxera resistance, nematode resistance, adaptability to high pH soils, saline soils, low pH soils, wet or poorly drained soils and drought. These effects take place in a more or less indirect manner and are consequences of the interactions between environmental factors and the physiology of the scion and rootstock cultivars employed[23]. Rootstocks have recently gained great importance in the only consistently effective and successful strategy in Egypt[18, 29]. Sometimes the graft union is unsuccessful, resulting in the main stem breaking off, dieback, poor growth or death of the top part of plant. In contrast, the root system often remains alive and may send up suckering shoots. Graft failure can be caused by factors such as poor formation of the graft union (due to problems with anatomical mismatching, poor grafting technique, adverse weather conditions and poor hygiene), mechanical damage to the graft union and graft incompatibility.[22] excluded phytopathogenic bacteria and phytoplasma infection as a possible cause for Syrah decline in French vineyards and reported that fungal infected graft unions is considered the main reason for graft failure. Other fungi, including Phomopsis spp., Verticillium spp. and Alternaria spp., and those involved with esca and Eutypa were found to be associated with the graft union of symptomatic plants; however these fungi also occurred in control plants, so that it was not possible to correlate them specifically with Syrah decline[21]. Also in Spain,[10] reported that species of Botryosphaeriaceae Ces. & De Not. were isolated from the grapevine rootstocks, the graft union and the scion cultivars and its frequency was 23.1, 61.5 and 61.5%, respectively. In addition,[1] proved that Phoma sp. caused dead-arm and die-back disease of grapevine in Bulgaria. In Egypt, Phomopsis viticola (Sacc.) and Botryodiplodia theobromae Pat. were reported to cause dead-arm and die–back diseases, respectively[3, 20]. In addition, Fusarium solani (Mart.) Sacc. can cause necrosis in xylem parenchyma and xylem vessels; however the author detected that Botryodiplodia theobromae, Phomopsis viticola and Fusarium solani can colonized the tissues with their hyphae in addition to the necrosis in xylem parenchyma and vessels[2]. For grafting material disinfection, pruning and grafting tools were disinfected with bleach (7% active chlorine)[32] or the disinfectant, hydroxyquinolene sulphate was highly effective at reducing germination but less effective against mycelial growth[33]. The main objective of this investigation was to determine the fungal species associated with graft union and their impact on grafting failure of various grapevine rootstocks (Dogridge, Saltcreek and Freedom) on some scion cultivars including Superior, Thompson and Flame Seedless. Studying different approaches for disease control methods was also conducted.
2. Materials and Methods
2.1. Experimental Sites
- The present study was performed during the seasons 2011-2012 in four different locations in Egypt including grapevines development project greenhouse, Giza (GDPG), grapevines development project greenhouse, Pahteem (GDPP), horticultural research institute greenhouse, Giza (HRIG), and in private greenhouse, Belbeis, Sharkeya governorate (PGB).
2.2. Preparation for Bench Grafting
- The operation conducted in this section was according to[9] with minor modification. During winter dormancy, in September, before any major winter damage has occurred to the wood or buds, one -years-old canes of each rootstock (Dogridge, Saltcreek and Freedom) and scion (Superior, Thompson and Flame Seedless) were cut into pieces 30-35 cm with 8-12 mm diameter and soaked overnight in clean water just to cover. One day after, the cutting were left in a sheltered area and covered with a wet tarp to be kept from drying and packed in polyethylene lined bags with peat moss and sealed for keeping moisture in the cutting and placed in a cold storage unit at 0±1°C.
2.3. Grafting
- Before grafting, pieces of the rootstocks were disbudded with a sharp knife, leaving the bottom buds un-removed. Grafting was done in January and scion wood was cut into pieces 4-5 cm long with one compound bud/each. The rootstock and scion were cut separately, then joined together and tying with poly-ethelene. The lower ends of the cuttings were dipped in indole-butyric acid at a concentration of 1000 ppm. Then placed in callus box with moisture medium consisting rice hay 1:1 and filled 3cm to below the graft cutting. After hardening, in February, the grafted plants were cultivated in planting poly-ethylene bags 15×15×30 cm3 with a medium consisting of peat moss:sand:rice hay 1:1:1. The plants were protracted, misted to keep humidity at 80% and temperature at 23-25°C. After four weeks, a fertilizer program started and the plants were pinched after four leaves top growth. The method of grafting used was cleft grafting.
2.4. Isolation Techniques
- During adaptation of grafting seedlings, we observed a large number of seedlings failure occurred in an area were grafting this region turned into a brown to black and oblique separation occurred between scion and rootstock. Also, seedlings showed some fungal cultures within the grafting area. The different fungal isolates used in the study were obtained from a combination of scions grafted onto rootstocks mentioned above. The collected samples of natural infected grafting area were thoroughly washed under running tap water, cut into small pieces (1 cm-long), and surface sterilized with dipping in 0.1% mercuric chloride solution for 2 minutes, then washed in several changes of sterile distilled water. The surface sterilized pieces were blotted dry on sterilized filter paper, and transferred individually to Petri dishes, each containing 20 ml potato dextrose agar (PDA) medium, then incubated at 25°C for 5 days and inspected for fungal growth. The developed fungal colonies were purified using hyphal tip and single spore techniques. The purified fungus were identified according to their morphological characteristics described by[4] and confirmed by Mycology and Plant Disease Survey Department, Plant Pathology Research Institute, ARC, Egypt.
2.5. Inoculation Techniques
2.5.1. Preparation of Spore Suspensions
- Fungal spores were harvested from 2-weeks old PDA cultures. An amount of 5 ml of sterile water, containing 0.05% (v/v) tween 80 (Sigma, St. Louis, MO) to improve the wetting properties of the solution, was added to a Petri plate culture, the spores were gently dislodged from the surface with a sterile glass rod, and suspensions were filtered through three layers of cheesecloth to remove mycelial fragments. Spore concentration was adjusted using a haemocytometer to obtain 106 conidia/ml.
2.5.2. Pathogenicity Test
- Bench grafting was prepared as previously described. However, scions and rootstocks were immersed on the spore suspensions for each fungus for 20 minutes before joined. Each treatment contains four replicates and each replicate consists of five grafted seedlings. The grafted seedlings were observed for one year, length of the growing season and final results recorded at the end of the year.
2.6. Chemical Control
- Six different fungicides namely Syllet 40% (SC), Billis 38% (WG), kemazed 50% (WP), Saprol 19% (DC), Conazol 10% (EC) and Topsin M 70 (WP) (Table 1) were tested in greenhouse condition to evaluate their efficacy against fungal pathogens. Grapevines cutting, collected from different vineyards as scions or rootstocks, soaked or dipped in fungicides for 20 minutes before dipping in the pathogens suspensions. Each treatment contains four replicates and each replicate consists of five seedlings. These fungicides applied three times in growing season, (i) after raise plastic cover from seedlings Fig. 2 (K&L), (ii) at the beginning of adaptation period and (iii) monthly treatment. Final results were recorded at the end of the growing season.
2.7. Biological Control
|
2.8. Statistical Analysis
- All experiments were set up in a complete randomized design. The obtained data were subjected to statistical analysis using L.S.D test at P < 0.05 level to differentiate the differences between various treatments.
3. Results and Discussion
3.1. Symptoms and Fungi Isolation
- Symptoms showed as a graft failure, rootstock and scion become partly or completely separated and corky, layer forms between the rootstock and the scion (Fig. 1 A, B, D), decay of the wood around and below the graft union also find a dark line or corky tissue following the contours of the union between the rootstock and scion (Fig. 1 E, C, F), mycelium of the pathogen fungi appeared in graft union and on other buds on the scion cutting (Fig. 1E).
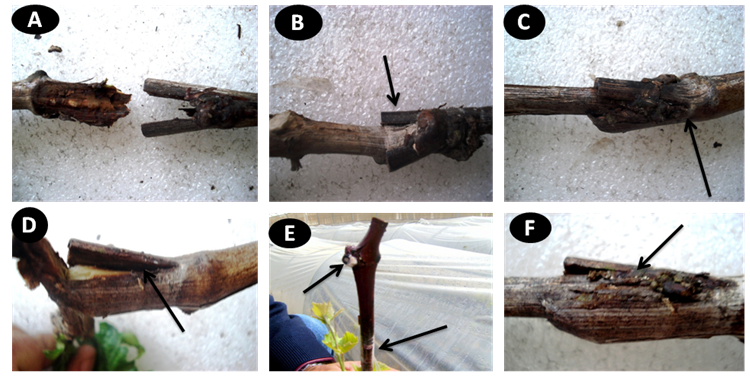 | Figure 1. (A&B&D) Rootstock and scion become partly or completely separated (C&F) corky layer forms between the rootstock and the scion, (E) decay of the wood around and below the graft union also find a dark line or corky tissue following the contours of the union between the rootstock and scion mycelium of the pathogen fungi showed in graft union and on other buds on scion cutting |
 | Figure 2. (H1) Phomopsis viticola, (H2) Botryodiplodia theobromae (H3) Alternaria solani (H4) Phoma sp. (H5) Fusarium solani (K&L). Raising plastic cover from seedlings at the beginning of adaptation period (M) Grafted seedlings under tunnel covered with plastic cover (I&J) Failure grafted seedlings |
|
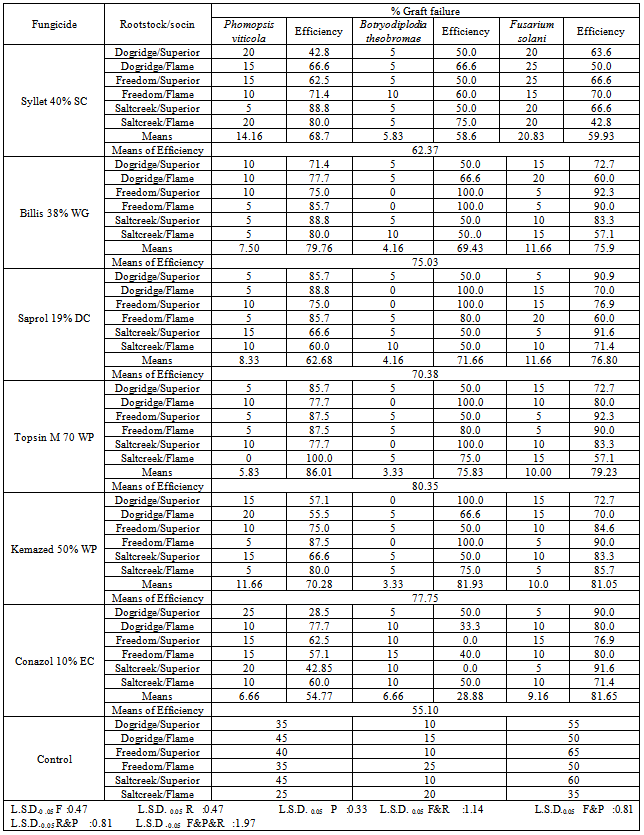
- Fungal pathogens were isolated from graft failure seedlings namely Phomopsis viticola, Botryodiplodia theobromae, Fusarium solani, Phoma sp. and Alternaria solani (Ellis and Martin) Jones and Grout. Frequency of the isolated fungi varied from location to another (Table 3 and Fig. 2H). In general, P. viticola was the most common isolated fungi (37.5%) on all localities followed by B. theobromae (34.5%). Whereas, the other isolated fungi were tiny such as Phoma sp., F. solani and A. solani recording 11.5, 10.3 and 6.3%, respectively. Actually, B. theobromae, P. viticola and F. solani caused necrosis in xylem parenchyma and xylem vessels as well as colonized the tissues with their hyphae. Dark inclusions bodies and abundant production of tyloses were clearly noticed.[2] cited the important role for such pathogenic fungi in order to exhibit graft failure and decay of grafting union.
3.2. Pathogenicity Test
- The results showed that Fusarium solani was recorded at low level (10.27%) but it gave a highest percentage of graft failure estimated to be about 52.5% in all permutations and combinations rootstock/scion were studied as Dogridge/Superior, Dogridge/Flame, Freedom/Superior, Freedom/Flame, Saltcreek/Superior, and Saltcreek/Flame. Moreover, the percentage of graft failure was 37.5, 15, 1.7, and 1.7% for Phomopsis viticola, Botryodiplodia theobromae, Phoma sp. Sacc. and Alternaria solani, respectively (Fig. 3). The species of Botryosphaeria were associated with wood and trunk diseases of grapevines in Portugal. Three species, namely, B. obtuse (Schw.) Shoemaker, B. Parva Pennycook & Samuels, and B. Lutea A.J.L. Phillips, were regularly associated with trunk dieback, wood necrosis, brown wood streaking, cane bleaching or incomplete grafts[19]. The author indicated that B. parva is associated with many of the symptoms normally linked with infection by other fungi in the grapevine decline syndrome. In the same manner, Botryosphaeriaceae have traditionally been considered wound pathogens[30]; however,[25] reported that these fungi also directly infected plants without wounds, penetrating through the lenticels, stomata and other natural openings. Many of the previous articles cited the ability of isolated pathogens herein to motivate grafting failure process.
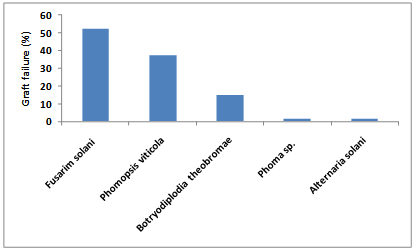 | Figure 3. Pathogenicity test by different isolated fungi on all permutations and combinations rootstock/scion. (L.S.D R 0.05:1.01, L.S.D. 0.05 P: 0.92, L.S.D. 0.05: R&P: 2.27) |
3.3. Chemical Control
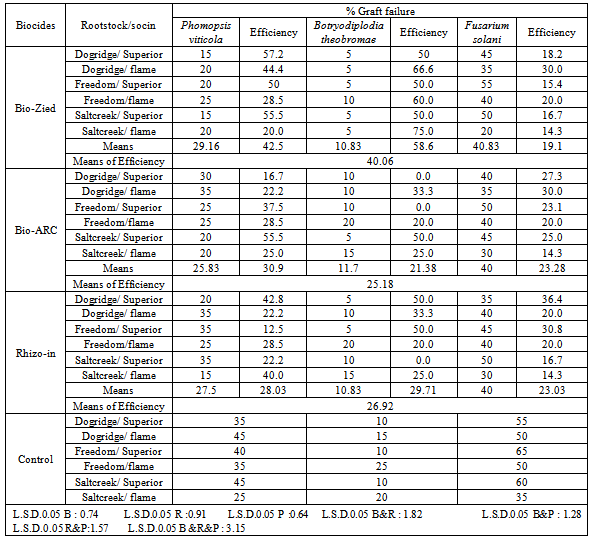
- Our results demonstrated that topsin M and kemazed were the most effective fungicides for controlling fungal pathogen cause graft failure. Considering the average of efficiency of tested fungicides against P. viticola, B. theobromae and F. solani, the percentage of reduction in graft failure incidence, was reduced by approximately 80 and 78%, respectively. Moreover, Billis, Saprol, Syllet and Conazol reduced the percentage of reduction by 75, 70, 62 and 55%, respectively (Table 4). The satisfactory activity of thiophanate – methyl (Topsin M70) and carbandazium (Kemazed) belonging to benzimidazole fungicides shown in the present study confirmed the previous finding for inhibition of spore viability and mycelia growth of P. viticola by benzimidazole fungicides, both in vitro[14, 28, 7, 20] and in vivo[6, 5, 13]. Recently, an attempt to control Petri disease pathogens in the grapevine propagation process, azoxystrobin, carbendazim and tebuconazole were the most effective fungicides against Phaeomoniella chlamydospora (W. Gams, Crous, M. J. Wingf. & L. Mugnai) Crous & W. Gams, while carbendazim and didecyldimethylammonium chloride were the most effective against P. aleophilum (W. Gams, Crous, M. J. Wingf. & L. Mugnai)[11]. Regarding to its mode of action, it is suggested that high effectiveness of systemic fungicides from this chemical group results from their ability of translocation into the plant tissues and destruction of the pathogens during the time of infection and incubation[7, 24]. However, the application of thiophanate – methyl should be limited to one treatment during the vegetative period because of the possibility to develop the resistance to these chemicals of wide range of fungal species[8]. Benzimidazole fungicides as Topsin M 70 and Kemazed affect mitosis and cell division of fungal pathogens. Meanwhile Saprol belonging to traiazol group and (Syllet) Dodine’s mode of action is through disruption of cell membranes. Systemic penetrates form a protective barrier on the plant, permeate into the plant, move upward in the plant's xylem, and move downward in the plant's phloem. These fungicides have protective activity including new growth and have good curative activity.
3.4. Biological Control
- The bio-fungicides found efficient in suppressing fungal pathogens cause graft failure on grapevine. The results showed that, the tested bio-fungicides reduced disease incidence when applied at the same time of pathogen inoculation. Considering the means of efficiency of the biological products against P. viticola, B. theobromae and F. solani, Bio-Zied (Trichoderma album) was the most effective one reducing the percentage of disease incidence by 40 %. In addition, the disease incidence was reduced by 27 and 25% when Rhizo-in (Bacillus subtilis) and Bio- Arc (Bacillus megaterium), respectively applied by the same method (Table 5). Its known that, biological control of plant diseases can occur through different mechanisms, which are generally classified as; antibiosis, competition, suppression, direct parasitism, induced resistance hypovirulence and predation[17, 16]. The antagonistic activity has often been associated with production of secondary metabolites[26]. At the same time, molecular markers provide gigantic sources can assist scientists in developing tools to monitor the genetic and environmental fate of these agents[27]. Besides other modes of action, enzymes responsible for cell-wall degradation such as chitinases and glucanases have been associated with the ability of Trichoderma to control plant pathogens[15]. Recently, phytoalexins proved to play an important role for the induction of resistance which should be considered as one of the possible modes of action of alternative control means in controlling fruit decay[31]. Depending on the results obtained herein, however, even commercially available products still need to be enhanced. A likely scenario is that the use of biological products in general will continue to increase but will complement or be combined with low risk chemical fungicides, natural antimicrobial substances and other physical methods. As a conclusion, phytopathogenic fungi can be considered one of the most important factors influencing graft union success of grapevine in Egypt.
|
|
ACKNOWLEDGEMENTS
- The authors are grateful to Prof. A.A. Shalaby in PPathRI, Prof. F. Al Morsy and Prof. Aisha S.A. Gaser in HRI, ARC for providing the facilities and advise to carry out this study.
References
| [1] | Abo Rahab M.E.A, 2002, Dead- arm disease Phomopsis viticola Scc. and its control in Bulgaria. PhD Thesis Agricultural University, Plovdive, Bulgaria. |
| [2] | Atia M., Aly A.Z., Tohamy M.R.A., El-Shimy H. and Kamhawy M.A., 2003, Histopathological studies on grapevine die- back. Journal of Plant Diseases and Protection, 110, 131-142. |
| [3] | Attia M.F. and Saber M.M., 1995, Dead-arm disease of grapes (Note). Egyptian Journal of Phytopathology, 23 (12), 109. |
| [4] | Barnett H.L. and Hunter B.B., 1986, Illustrated genera of imperfect fungi. 4th Ed., Macmillan Publishing Co., New York, 218 pp. |
| [5] | Berezovskaja E.A. and Vološina N.P., 1987, Sistema borby s ernoj pjatnistostju vinograda. Informacionnyj listok, 31, 1-3. |
| [6] | Bourbos V.A. and Shourdridakis M.T., 1991, Efficacy of some fungicides against excoriosis of grape. Revue Suisse de Viticulture Arboriculture Horticulture, 23, 197-198. |
| [7] | Castillo-Pando M.S., Nair N.G., Emmett R.W. and Wicks T.J., 1997, Inhibition in pycnidial viability of Phomopsis viticola on canes in situ as an aid to reducing inoculum potential of cane and leaf blight disease of grapevines. Australasian Plant Pathology, 26, 21-25. |
| [8] | Czerniakowski Z.W. and Czerniakowski Z., 1999, Nowe środki ochrony roślin. AR im. H. Kołłątajaw Krakowie, WODR- Boguchwała, Rzeszów, 131 pp. |
| [9] | Gaser A.S.A., 2007, Impact of some Rootstocks on Performance of Superior Grape Cultivar. J. Agric. Sci. Mansoura Univ., 32 (11), 9155-9183. |
| [10] | Gramaje D., Muñoz R.M., Lerma M.L., García-Jiménez J. and Armengol J., 2009, Fungal grapevine trunk pathogens associated with Syrah decline in Spain. Phytopathologia Mediterranea, 48, 396-402. |
| [11] | Gramaje D., Aroca A., Raposo R., García-Jiménez J. and Armengol J., 2009, Evaluation of fungicides to control Petri disease pathogens in the grapevine propagation process. Crop Protection, 28, 1091-1097. |
| [12] | Online Available:http://faostat.fao.org/site/567/DesktopDefault.aspx?PageID=567#ancor |
| [13] | Król E., 2005, Influence of some chemicals on the viability of Phomopsis viticola SACC.spors. Journal of Plant Protection Research, 45(3), 195-203. |
| [14] | Kuropatwa E., 1994, Badanie efektywności grzybobójczej fungicydów dla Phomopsis viticola Sacc. powodujacego nekrozę korową winorośli. Ann. Univ. Mariae Curie-Skłodowska, Sect. EEE – Horticultura, 2, 109-115. |
| [15] | Matroudi S., Zamani M.R. and Motallebi M., 2009, Antagonistic effects of three species of Trichoderma sp. on Sclerotinia sclerotiorum, the causal agent of canola stem rot. Egyptian Journal of Biology, 11, 37-44. |
| [16] | Morsy K.M., Abdel-Monaim M.F. and Mazen M.M., 2011, Use of Abiotic and Biotic Inducers for Controlling Fungal Diseases and Improving Growth of Alfalfa. World Journal of Agricultural Sciences, 7, 566-576. |
| [17] | Moyer C. and Peres N.A., 2008, Evaluation of bio fungicides for control of powdery mildew of gerbera daisy. Proceedings of the Florida State Horticultural Society, 121: 389-394. |
| [18] | Omer A.D., Granett J., Kocsis L. and Downie D.A., 1999, Preference and performance responses of California grape phylloxera to different Vitis rootstocks. Journal of Applied Entomology, 123, 341-346. |
| [19] | Phillips A.J.L., 2002, Botryosphaeria species associated with diseases of grapevines in Portugal. Phytopathologia Mediterranea, 41, 3-18. |
| [20] | Rashed M.F, Kamhawy M.A.M.and Abo Rehab M.E.A., 2006, Histopathological and control of Grapevine Dead-arm Disease. J. Agric. Sci. Mansoura Univ., 31(5), 2815-2824. |
| [21] | Renault-Spilmont A.S. and Bourisquot J.M, 2002, Syrah Decline in French vineyards. FPMS Grape Program Newsletter. Foundation Plant Materials Service, University of California, Davis. CA, USA, 22–23. |
| [22] | Renault-Spilmont A.S., Grenan S. and Bourisquot J.M., 2007, Syrah decline in French vineyards: Rootstocks and Syrah clone impact, pathological and genetic studies. Proceedings of the Syrah Vine Health Symposium. University of California, Davis, CA, USA, 5-7. |
| [23] | Reynolds A.G. and Wardle D.A., 2001, Rootstocks impact vine performance and fruit composition of grapes in British Columbia. Hort. Technol. 11, 419-427. |
| [24] | Schüepp H. and Siegfried W., 1988, Auftrefen, Bedeufung und Bekämpfung der Pilzkrankheiten im Rebbou der deutschprachigen Schweiz. Gesunde Pflanzen, 40, 286-294. |
| [25] | Slippers B. and Wingfield M.J., 2007, Botryosphaeriaceae as endophytes and latent pathogens of woody plants: diversity, ecology and impact. Fungal Biology Reviews, 21, 90-106. |
| [26] | Sliva G.H., Costa V.P., Campos V.P., Oliverira D.F. and Pfenning L.H., 2001, Fungal metabolites with activity against nematodes. Bioactive Fungal metabolites. Impact and Exploitation, International Symposium. Br. Mycolog. Soc., Wales Swansea, UK, 95 pp. |
| [27] | Spadaro D. and Gullino M.L., 2005, Improving the efficacy of biocontrol against soilborne pathogens. Crop Protection, 24, 601-613. |
| [28] | Todorova M. and Katerova L., 1995, Infuence of some fungicides on Phomopsis viticola Sacc., the agent of the dead-arm disease of grapevines. I. Influence on the mycelium growth. Rasteniev dni Nauki, 32, 211-213. |
| [29] | Troncoso A., Atte C.M. and Cantos M., 1999, Evaluation of salt tolerance of in vitro grown grapevine rootstock varieties. Vitis, 38(2), 55-60. |
| [30] | Van Niekerk J.M., Fourie P.H., Halleen F. and Crous P.W., 2006, Botryosphaeria spp. as grapevine trunk disease pathogens. Phytopathologia Mediterranea, 45, S43–S54. |
| [31] | Youssef K., Sanzani S.M., Ligorio A., Terry L.A. and Ippolito A., 2011, Biochemical changes associated with induced resistance on citrus fruit. Journal of Plant Pathology, 93 (4 supplement) S4, 22. |
| [32] | Serra S., Mannoni M., Ligios V. and Fiori P.P. 2011. Occurrence of Phaeomoniella chlamydospora on grapevine planting material in Sardinia and its control with combined hot water and cyproconazole treatments. Phytopathologia Mediterranea, 50 (Supplement), S61−S76. |
| [33] | Jaspers, M.V. 2001. Effect of fungicides, in vitro, on germination and growth fo Phaeomoniella chlamydospora. Phytopathologia Mediterranea, 40 (Supplement): S453-S458. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML