-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2013; 3(2): 34-39
doi:10.5923/j.ijaf.20130302.02
Aldehydes Compounds for Controlling Black Scurf Disease of Potato (Solanum Tubrosum L.) under Field Conditions
Mohamed A. Abd-Alla, Nehal S. El-Mougy, Mokhtar M. Abd-El- Kader, Farid Abd-El-Kareem, Nadia G. El-Gamal, Ried S. R. El- Mohamedy
Plant Pathology Department, National Research Centre, El-Behouth St. Dokki 12622, Giza, Egypt
Correspondence to: Nehal S. El-Mougy, Plant Pathology Department, National Research Centre, El-Behouth St. Dokki 12622, Giza, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
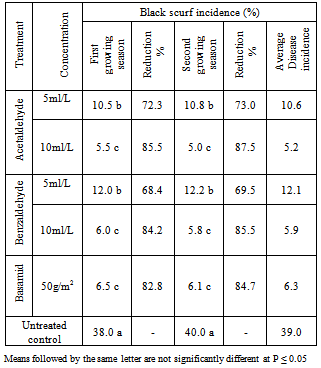
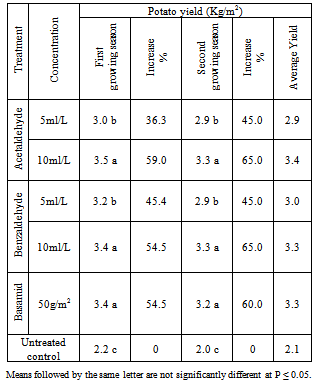
Field trials were carried out for evaluating the efficacy of Acetaldehyde and Benzaldehyde soil application on black scurf disease of potato plants. Laboratory tests revealed that all treatments significantly reduced the linear growth of Rhizoctonia solani. Acetaldehyde and Benzaldehyde solutions at concentrations of 4.0 ml/L completely inhibited the linear growth of Rhizoctonia solani in vitro. Acetaldehyde and Benzaldehyde at concentrations of 5.0 and 10.0 ml/L in addition to fungicide (Basamid) at 50 g/m2 of soil) were applied under field conditions to study their effect on black scurf disease and tuber yield of potato plants. Results indicate that that all treatments significantly reduced the disease incidence of potato plants. The highest reduction was obtained with Acetaldehyde and Benzaldehyde at concentrations of 10.0 ml/L and fungicide Basamid which reduced the disease incidence more than 85.0, 84.2 and 82.9% respectively during two successive growing seasons. Acetaldehyde and Benzaldehyde at concentrations of 5.0 ml/L reduced the black scurf incidence by 72.3 and 68.4% respectively. As for potato yield the highest increased was obtained with Acetaldehyde and Benzaldehyde at concentrations of 10.0 ml/L and fungicide Basamid which increased the tuber yield more than 59.0, 54.4 and 54.4% respectively during two successive growing seasons. Acetaldehyde and Benzaldehyde at concentration of 5.0 ml/L increased the potato yield by 36.5 and 45.0%, respectively.
Keywords: Acetaldehyde, Benzaldehyde, Black Scurf Disease, Potato, R. Solani
Cite this paper: Mohamed A. Abd-Alla, Nehal S. El-Mougy, Mokhtar M. Abd-El- Kader, Farid Abd-El-Kareem, Nadia G. El-Gamal, Ried S. R. El- Mohamedy, Aldehydes Compounds for Controlling Black Scurf Disease of Potato (Solanum Tubrosum L.) under Field Conditions, International Journal of Agriculture and Forestry, Vol. 3 No. 2, 2013, pp. 34-39. doi: 10.5923/j.ijaf.20130302.02.
Article Outline
1. Introduction
- Soil borne diseases are still a major threat to potato cultivation in Egypt. Several important potato pathogens in Egypt originated from soil borne inoculums. Potato plants are susceptible to devastation by various diseases such as Black scurf caused by Rhizoctonia solani and dry rot caused by Fusarium sambucinum[1, 2, 3].Potato plants (Solanum tuberosum L.) considered one of the most important vegetable crops overall the world. Rhizoctonia diseases of potato are caused by the fungus Rhizoctonia solani Kühn can be found on all underground parts of the plant at different times during the growing season. Rhizoctonia solani causes black scurf on tubers, stem and stolen canker on underground stems and stolens and occurs wherever potatoes are grown. The symptoms of the disease are found on both above and below ground portions of the plant. Black scurf is the most conspicuous sign of Rhizoctonia disease[4, 5]. In this phase of the disease the fungus forms dark brown to black hard masses on the surface of the tuber. These are called sclerotia, the resting bodies of the fungus. Although black scurf is the most noticeable sign of Rhizoctonia, stem canker, is the most damaging of the disease as it occurs underground and often goes unnoticed[6, 7]. Early in the season, the fungus attacks germinating sprouts underground before emerging shoots above soil surface. The sprout may be killed outright if lesions form near the growing tip. Damage at this stage results in delayed emergence and is expressed as poor and uneven stands with weakened plants[7].Controlling such disease mainly depend on fungicides treatments[8],but fungicides application cause hazards to human health and increase environmental pollution such as toxic residues and selection of resistant isolates of the pathogen[9, 10, 11]. Therefore, alternative treatments for control of plant diseases are needed. The search for safer phytochemicals with lower environmental and mammalian toxicity is a major concern and imperative[12, 13]. Acetaldehyde and Benzaldehyde produced by fruits during ripening showed antifungal activity. Acetaldehyde has been found effective in postharvest protection of apples, sweet cherries and stone fruits[14, 15, 16]. Also, Acetaldehyde has been tested against decay microorganisms commonly found on strawberry fruit such as Botrytis cinerea and Rhizopus stolonifer[17]. Damage at this stage results in delayed emergence and is expressed as poor and uneven stands with weakened plants[16, 17].Some plant volatiles, e.g. acetaldehyde, Benzaldehyde, Cinnamaldhyde, ethanol, benzyl alcohol, nerolidol, 2-nonanone have also been found to have antifungal activity against the fruit and vegetable pathogens Penicillium digitatum, Rhizopus stolonifer, Colletotrichum musae and Erwinia caratovora during in vitro trials. Certain plant volatile aldehydes i.e. Acetaldehyde has also been reported to inhibit postharvest microorganisms such as Erwinia carotovora, Pseudomonas fluorescens, Monilinia fructicola, Penicillium spp. and various species of yeast commonly found on fruit and vegetables[18, 19]. Benzaldehyde has been used fumigating peaches to protect them against Rhizopus rot, and it is also totally inhibited spore germination of Botrytis cinerea and germination of Monilinia fructicola[16, 18, 19, 20]. The main objectives of the present research were designed to study the efficacy of Acetaldehyde and Benzaldehyde on the mycelial growth of Rhizoctonia solani as well as their application as soil treatment against black scurf incidence of potato plants under field conditions and its influence on tuber yield.
2. Materials and Methods
2.1. Pathogen, Plant Material and Chemicals
- Different isolates of Rhizoctonia solani were isolated from diseased potato tubers samples collected from different fields located at Nubaria region, Behera Governorate, Egypt. This work was done by the authors of the present work. The isolated fungi were tested for their ability to induce Black scurf disease of potato and proved their pathogenicity in this regard. The highly aggressiveness isolate was used in the present study. Potato seeds cv. Diamond were used for cultivation under field conditions. Pure active ingredients of Acetaldehyde and Benzaldehyde (RAMADA Co., Egypt were used. These chemicals were kept in a refrigerator at 5°C till use.
2.2. Laboratory Tests
- The inhibitor effect of volatile aldehyde solutions at different concentrations against the linear growth of Rhizoctonia solani was evaluated in vitro. The aldehydes concentrations of 0.50, 1.0, 2.0 and 4.0 ml/L were tested. A different certain volumes of aldehydes were added to flasks containing sterilized PDA medium before its solidifying to obtained the proposed concentrations, then poured in Petri dishes. Another set Petri dishes containing PDA aldehyde-free medium were kept as control treatment. Disks (6- mm-diameter) taken from the edge of fungus culture were placed in the centre of each Petri plates. Five Petri plates were used as replicates. All Petri plates were incubated at 25 ± 1°C. Fungus linear growth was measured when the control plates reached full growth, then the average diameter of linear growth was calculated and the percentage of reduction in mycelial growth was calculated for aldehydes concentrations relative to the control treatment. This test was repeated three times.
2.3. Field Experiments
- Experiments were carried out at the Researches and experimental station (NRC) in Nubaryia region, Behera Governorate, Egypt for two successive growing seasons. The influence of aldehyde solutions application against black scurf disease incidence and potato tuber yield was determined during the two successive growing seasons. Field experiments consisted of plots (4x8 m) each comprised of 8 rows and 32 holes / row was conducted in a Complete Randomized Block design with three replicates (plots) for each particular treatment.Treatments: Acetaldehyde and Benzaldehyde solutions at two concentrations i.e. 5.0 and 10.0 ml/L, in addition to the fungicide (Basamid) at 50 g/m2 of soil) were applied before potato cultivation.Application: All plots were irrigated to full water holding capacity. Three days later, the irrigated plots were sprayed with acetaldehyde and Benzaldehyde solutions as well as the fungicide at proposed concentrations at the ratio of 10 L/m2, then covered with polyethylene sheets for another three days. All plots were cultivated with potato seeds cv. Diamond, three days after polyethylene sheets removal.Disease assessment and yield determination: Percent of diseased plant was recorded up to 90 days of planting. Tuber yield of potato (kg/m2) for each treatment was also determined. All the previous procedures were repeated twice at two successive growing seasons at the same area.
2.4. Statistical Analysis
- One way analysis of variance (ANOVA) was used to analyze differences between inhibitor concentrations of Acetaldehyde, Benzaldehyde and the linear growth of R. solani as well as differences between Acetaldehyde, Benzaldehyde, Basamid and root rot incidence; produced yield at different applied concentrations under laboratory (in vitro) and field conditions (in vivo). MSTAT-C program (V2.1) was used to perform the analysis of variance. Turkey test for multiple comparisons among means was utilized[21].
3. Results and Discussion
3.1. Laboratory Tests
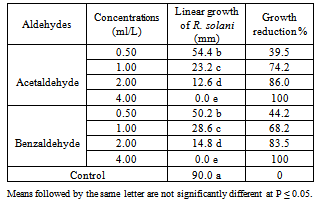
- The effect of increased concentrations of Acetaldehyde and Benzaldehyde on the growth of the fungus R. solani is presented in Table (1). Obtained results showed that Acetaldehyde and Benzaldehyde were significantly able to reduce gradually the linear fungal growth by increasing their concentrations. Complete growth inhibition of R. solani, was observed at concentration of 4.0 ml/L of both aldehydes tested. Meanwhile, the fungal growth reduction as (39.5, 44.2%); (74.2, 68.2%) and (86.0, 83.5%) was obtained with Acetaldehyde and Benzaldehyde treatment at 0.5, 1.0 and 2.0 ml/L, in respective order.
|
3.2. Field Experiments
|
|
ACKNOWLEDGEMENTS
- This work was supported financially by the National Research Centre Fund (NRC), Egypt, Grant No. 9050204.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML