-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2013; 3(1): 1-6
doi:10.5923/j.ijaf.20130301.01
Use of Some Plant Essential Oils as Post-harvest Botanical Fungicides in the Management of Anthracnose Disease of Mango Fruits (Mangi Feraindica L.) Caused by Colletotrichum Gloeosporioides (Penz)
M. A. Abd-AllA , Wafaa M. Haggag
Department of Plant Pathology, National Research Centre, Egypt
Correspondence to: Wafaa M. Haggag , Department of Plant Pathology, National Research Centre, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Mango suffers from several diseases at all stages of its life. Anthracnose, caused by the fungus Colletotrichum gleosporioides is the most important post harvest disease of mango. The aim of this study was to test the possibility of the use of some plant essential oils i.e. Basel oil (Ocimum basilicum), Orange oil (Citrus sinensis), Lemon oil (Citrus Medica) and Mustard oil (Brassica juncea L.) to reduce postharvest losses induced by Colletotrichum gloeosporioides (Penz.) in mango fruits. In this study, the antifungal activity of essential oils under in vitro condition were assayed by tested various concentrations (0, 50,100 and 150µg/ml)) and under in vivo condition by used different essential oil concentrations (0, 250,500 and 1000ppm) on inoculated mango fruits. Results of the present study showed that orange oil at all tested concentrations were a significant reducing the fungal linear growth if compared with other tested essential oils. At low concentration 50 (µg/ml) orange oil caused 10.0% reduction in fungal growth, while at 100 (µg/ml) caused 72.2% and at high tested concentration 150 (µg/ml) caused a complete reduction in mycelium linear growth of pathogenic fungus. Meanwhile, at low tested concentration 50 (µg/ml), mustard oil caused a highly significantly reduction of the percentage of fungal spore germination by 70.8 % followed by basil oil by 64.7%. Results of in vivo studies showed that, at low concentration 250 ppm, mustard oil caused a highly reduction of anthracnose incidence of mango fruits by 79.9% followed by basil oil with 66.7% .On the other hand, orange and lemon oil at low concentration (250ppm) were showed a highly effect to reducing the percentage of rotting fruit tissue by 84.5 and 75.0%, respectively if compared with other treatments and un-treated fruits.
Keywords: Plant Essential Oils, Mango Fruits, Anthracnose Disease, Colletotrichum Gloeosporioides
Cite this paper: M. A. Abd-AllA , Wafaa M. Haggag , Use of Some Plant Essential Oils as Post-harvest Botanical Fungicides in the Management of Anthracnose Disease of Mango Fruits (Mangi Feraindica L.) Caused by Colletotrichum Gloeosporioides (Penz), International Journal of Agriculture and Forestry, Vol. 3 No. 1, 2013, pp. 1-6. doi: 10.5923/j.ijaf.20130301.01.
Article Outline
1. Introduction
- Mango (Mangifera indica L.) is one of the top five fruit crops in the world. It is adaptable to a wide range of climates, ranging from wet tropical to dry subtropical. Among the various constraints, the most important is anthracnose caused by Colletotrichum gloeosporioides Penz. And Sacc. (teleomorph Glomerellac ingulata). Flower blight, fruit rot, and leaf spots are among the symptoms of this disease[2]. Severe infection destroys the entire inflorescence resulting in no setting of fruits. Young infected fruits develop black spots, shrivel and drop off. Fruits infected at mature stage carry the fungus into storage and cause considerable loss during storage, transit and marketing[1]. Disease control methods include the prophylactic use of fungicides such as benomyl, mancozeb, carbendazim, and thiabendazol[18]. The postharvest use of chemicals as fungicides is restricted in most countries[24]. Consumer demand for agricultural commodities without pesticide residues is high[7,24] however, pesticides may also kill various beneficial organisms and their toxic forms may persist in soil[12] and increase the incidence of resistance among pathogens towards synthetic chemicals[3,23]. Thus, a new preservation technologies are needed, which have to be considered as human-safe and environmentally friendly[9]. Among the various alternatives, natural plant products, including essential oils that are biodegradable and eco-friendly, are catching the attention of scientists worldwide. Such products from higher plants are bio-efficacious, economical, and environmentally safe and can be ideal candidates for use as agrochemicals[14]. Numerous reports showed that oils from some plant species are harmful to fungal pathogens[17]. Wilson et al.,[30], tested 49 essential oils from various plants and found that the oils from palmarosa (Cymbopogon martini), red thyme (Thymus zygis),cinnamon leaf (Cinnamomum zeylanicum), and clove buds (Eugenia caryophyllata) were effective in the control of Botrytis cinerea. Oils from Eucalyptus globules and Ocimum canum at 2000 ppm were effective in reducing mycelial growth and sclerotial production of Sclerotium rolfsii[27]. This study aimed to evaluate the effectiveness of a number of plant essential oils as alternative method against growth and spore germination of Colletotrichum gloeosporioides the causal agent of anthracnose disease of mango fruits under vitro conditions , and study their effect on the disease incidence under vivo conditions.
 | Figure 1. Anthracnose disease symptoms on mango fruits |
2. Materials and Methods
2.1. Fruits
- Mango (Mangifera indica L.) fruits Zabdia cv. were harvested at the mature stage, and sorted based on size and the absence of physical injuries or disease infection. Before treatments, fruit were surfaced disinfected with 2% sodium hypochlorite for 3 min, then rinsed with tap water, and air-dried.
2.2. Pathogen Culture
- Colletotrichum gloeosporioides was cultured for 1–2 weeks on potato dextrose agar (PDA) at 25 ◦C. The isolate used was obtained from infected mango fruit in Egypt. Spores were harvested by adding 3–4 ml of sterile, de-ionized water (diH2O) to the Petri dish. The spores were then rubbed with a sterile glass rod to free them from the PDA medium, and the spore suspension was passed through two layers of cheese cloth. The suspension was diluted with water to obtain the spore concentrations (106spores ml−1) according to determination with a Haemocytometer slid.
2.3. Source of Tested Plant Essential Oils
- Pure-grade (not containing synthetic chemicals and/or non-natural components) essential oils of Basel oil (Ocimum basilicum), Orange oil ( Citrus sinensis), Lemon oil(Citrus Medica) and Mustard oil (Brassica juncea L.), were obtained from CairoCompany for Oils and Aromatic Extractions CID, Egypt. These essential oils were stored in dark bottles at 4ºC for further studies.
2.3.1. In Vitro Screening of Plant Essential Oils Against C. Gloeosporioides Mycelium Linear Growth and Spore Germination
- The antifungal tests were carried out in vitro according to the method described by Pitarokili et al.[22] using Petri dishes 9 cm in diameter containing potato dextrose agar (PDA). The essential oils were dispersed individually as an emulsion in sterilized water using Tween 20 (0.05%) and added to PDA immediately before it was filled into the Petri dishes at a temperature of 45-50°C. The concentrations tested were0, 50,100 and 150µg/ml. The controls included the same quantity of Tween 20 mixed with PDA. The tested fungus was inoculated immediately after preparation of the Petri dishes by placing in the centre of each plate a 5 mm diameter disk of the test fungus, cut with a sterile cork borer from the periphery of actively growing cultures on PDA plates. The Petri dishes were incubated in the dark at a temperature of 25°C. Mean growth rates were calculated from five replicates of fungus every 24 h until fungal growth in the control filled the Petri dishes completely, the percentage mycelial inhibition was calculated by the following formula:% mycelial inhibition = dc _ dt X100dc: where dc is mean colony diameter of control sets and dt is mean colony diameter of treatment sets conidial germination inhibition test was performed by the cavity slide technique and the results were expressed in percentage[6].
2.3.2. In Vivo Applicability of the Plant Essential Oils of Anthracnose Incidence of Mango Fruits
- The Mango Fruits cv. Zabdeia were treated with different concentrations of plant essential oils by the standard techniques followed by Chandra[4] and Sharma & Yadav[25] in order to find out the efficacy of the oils against anthracnose rot disease caused by C. gloeosporioides. Mature healthy mango fruits of medium size were used for the experiment. The fresh mango fruits of control as well as of treatment sets were washed in running water and were surface sterilized with 0.1% sodium hypochlorite solution and were then washed with distilled water. Fruit inoculation with C. gloeosporioides spores obtained from a 7 day old culture, spores was suspended in sterile distilled water and 0.03% Tween- 80. Mango fruits were wounded on two sides to a depth of 1.5 mm by puncturing them with a pin. Each wound site was then inoculated with 40µl of spore suspension (106 spores/ml) of C. gloeosporioides , and kept at ambient temperature for drying. Air-dried fruits were dipped for 2-3 min in different concentrations of tested essential oils (250,500 and 1000ppm) individually and again kept at ambient for drying. After application of treatments mango fruits were packed in cardboard boxes and stored (13±1°C, 80± 5% RH) for 28 days. The effect of various plant essential oils on disease incidence and disease severity (percentage of fruit rotting tissue) was evaluated weekly for 28 days during cold storage. Six replicates were kept for treatment and control sets.
3. Statistical Analysis
- Tukey test for multiple comparisons among means was utilized (Neleret al.,1985).
4. Results
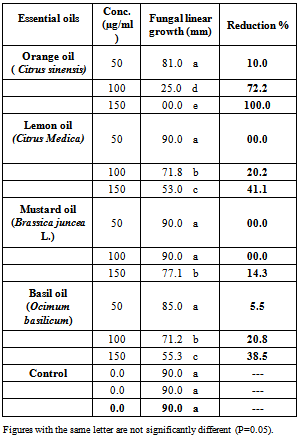
4.1. In Vitro Screening of Plant Essential Oils Against C. Gloeosporioides Mycelium Linear Growth
- Results in Table (1) showed that, orange oil at all tested concentrations was a significant reducing the fungal linear growth if compared with other tested essential oils . At low concentration 50 (µg/ml) orange oil caused 10.0% reduction in fungal growth, while at 100 (µg/ml) caused 72.2% and at high tested concentration 150 (µg/ml) caused a complete reduction in mycelium linear growth of pathogenic fungus. On the other hand, basil oil gave a moderate effect to reducing the fungal linear growth at all tested concentrations by 5.5, 20.8 and 38.5%, respectively. Lemon and mustard oil showed a lower effect at all tested concentrations if compared with the other oils and control treatments.
|
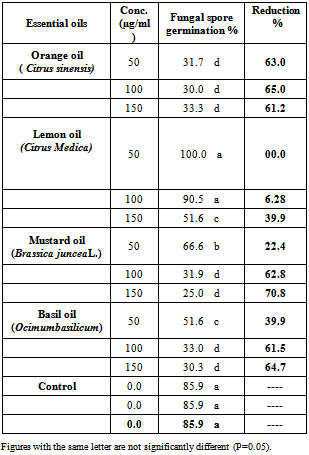
4.2. In Vitro Screening of Plant Essential Oils Against C. Gloeosporioides Spore Germination
- Results in Table (2) showed that, at low tested concentration 50 (µg/ml) ,mustard oil caused a highly significantly reduction of the percentage of fungal spore germination by 70.8 % followed by basil oil by 64.7% and orange oil by 61.2%. Meanwhile, at high tested concentration 150 (µg/ml) , orange oil caused 63.0% reduction of fungal spore germination , followed by basil oil by 39.9% and mustard oil by 22.4% . Lemon oil showed a lower effect to reducing the fungal spore germination than other tested oils at all tested concentrations.
|
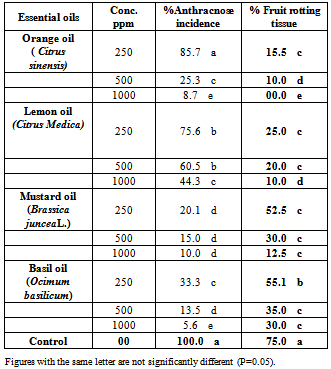
4.3. In Vivo Applicability of the Plant Essential Oils of Anthracnose Incidence of Mango Fruits
- Results in Table (3) and Fig.2. showed that , at low concentration 250 ppm , mustard oil caused a highly reduction of anthracnose incidence of mango fruits by 79.9% followed by basil oil with 66.7% reduction of disease incidence, while orange oil and lemon oil showed a moderate effect . At high concentration 1000 ppm, basil oil caused 94.4% reduction of disease incidence followed by orange and mustard oils by 91.3 and 90.0 %, respectively. On the other hand, orange and lemon oil at low concentration (250ppm) were showed a highly effect to reducing the percentage of rotting fruit tissue by 84.5 and 75.0%, respectively if compared with other treatments and un-treated fruits. Mustard and basil oils showed moderate effect at all tested concentrations.
 | Figure 2. Effect of mango fruits coating with various concentrations of plant essential oils on anthracnose disease incidence after 15 days of storage |
5. Discussion
- The essential oils are reported to have some fungicidal properties against certain postharvest diseases of tropical fruits and vegetables[30,16,13] and are also safer for the environment than synthetics chemicals .The ability of four plant essential oils to inhibit the fungal growth and their effect on spore germination of C. gloeosporioides was evaluated. The most active oils for reducing the growth of tested fungi with significant value under vitro condition was orange oil followed by basil oil, Lemon and mustard oil showed a lower effect at all tested concentrations if compared with the other oils and control treatments. On the other hand, at low tested concentration 50 (µg/ml) ,mustard oil caused a highly significantly reduction of the percentage of fungal spore germination by 70.8 % followed by basil oil by 64.7% and orange oil by 61.2% .The literature is also silent on the mode of action of the essential oils when used as postharvest fungi toxicants[29]. A substance may inhibit the growth of fungi either temporarily (fungistatic) or permanently (fungicidal). In agreement with the findings, Thangavelu et al.[28] found that the extracts of Solanumtorvum, Jatrophagl and ulifera and Emblica officinalis were highly inhibitory to mycelial growth of Colletotrichu mmusae in banana and the inhibitory effect was directly related to the quantity of extract added to the medium. In another study by Palhano et al.[21] also confirmed that the inhibitory effects of citral on spore germination of C. gloeosporioides were higher with an increase in the concentration of essential oil. In vivo applicability of the plant essential oils of anthracnose incidence of mango fruits was indicated that, At high oil concentration 1000ppm, coating mango fruits by basil oil caused 94.4% reduction of disease incidence followed by orange and mustard oils by 91.3 and 90.0 %, respectively. On the other hand, mango fruits were coating with orange and lemon oil at low concentration (250ppm) were showed a highly effect to reducing the percentage of rotting fruit tissue by 84.5 and 75.0% , respectively if compared with other treatments and un-treated fruits. Maqbool et al., 2010, reported that, Cinnamon oil with fungitoxic or fungistatic activity could be considered as a suitable alternative to synthetic fungicides for managing anthracnose in bananas. In vitro inhibition was directly related to the cinnamon oil concentrations. Dubey et al.[8] described that essential oil from Eupatorium cannabinum had an antifungal activity against Botryodiplodia theobromae and Colletotrichum gloeosporioides causing stem end rot and anthracnose diseases in mango, respectively. In addition, they found that this essential oil had an inhibitory effect on pectinase and cellulase, two important enzymes produced byphytopathogenic fungi in disease development. Farrag et al. [10] described that the antimicrobial activity of essential oils could be related to the presence of an aromatic nucleus and OH group that can affect hydrogen bonds of enzymes in microorganisms. Feng and Zheng , 2007, studied the effects of cassia oil on decay development in artificially inoculated and wounde tomatoes fruits. The results indicate that when wounded cherry tomatoes were treated with cassia oil, all concentrations (except 100 ppm) significantly inhibited A. alternateon tomatoes stored at 20 _C for 5 days (p < 0.05). The percentages of decayed cherry tomatoes treated by 500 ppm cassia oil was reduced by 34.2% compared to the control. Maqbool et al., 2010,reported that, different concentrations of cinnamon oil not only delayed the onset of anthracnose disease in coating panama fruits but also maintained the freshness during first two weeks of storage and later on showed minimal symptoms. The highest fungicidal effect was observed in those bananas treated with 0.4% cinnamon oil (disease incidence of 8.0%) and disease severity (DS) score of 1.2 indicating fruit surface infection close to 1.0. Shelef[26] described that within several components available in essential oils, the antimicrobial activity of phenolic compounds were higher than alcoholic components, this is in agreement with the results of present study. It is known that the cell wall of pathogens is the main target of phenolic compounds and these compounds may disrupt the permeability barrier of cell membrane and inhibit respiration. Hydrophobic nature of essential oils and their components enables these compounds to penetrate lipid of fungal cell membrane and mitochondria as a result disturbing their structure[5] and these compounds accumulate in the cell membrane of pathogen causing energy deletion. In addition, in some studies, it is reported that the essential oils may affect the metabolic pathways of microorganisms. Nychas[20] found that phenolic compounds in low concentration disrupt proteins and in high concentrations damaged the enzymes outbreak in production of energy.
6. Conclusions
- The results of present study showed that the possibility of the use of some plant essential oils i.e. Basel oil (Ocimum basilicum), Orange oil (Citrus sinensis), Lemon oil (Citrus Medica)and Mustard oil (Brassica juncea L.)to reduce postharvest losses induced by Colletotrichumgloeosporioides (Penz.) in mango fruits. So essential oils can be usedas a potential source of sustainable eco-friendly botanical fungicides, after successful completion of wide range trials.
ACKNOWLEDGMENTS
- This manuscript funded from the project “New applied approaches to promote productivity and Quality of some fruit crops (Mango)” National Research Centre, 2007 to 2010.
References
| [1] | Abd-AllA , M.A.and Wafaa M. Haggag,2011.New Safe Methods for Controlling Anthracnose Disease of Mango (Mang iferaindica L.) Fruits Caused by Colletotrichum gloeosporioides (Penz.)Journal of American Science 7, 80-86 |
| [2] | Arauz, L.F. 2000. Mango anthracnose: Economic impact and current options for integrated management. Plant Dis. 84: 600-611 |
| [3] | Cakir,A. S. Kordali, H. Kilic and E. Kaya, 2005. Antifungal properties of essential oil and crude extracts of Hypericum linarioides Bosse, Biochemical Systematics and Ecology 33: pp. 245–256. |
| [4] | Chandra, H. 1984.Evaluation of some higher plants for their volatile activity against blue mould rot of orange. PhD. Thesis, Gorakhpur University. Gorakhpur, India. |
| [5] | Cox, S.D., Mann, C.M., Markham, J.L., Bell, H.C., Gustafson, J.E., Warmingt on, J.R., Wyllie, S.G., 2000. The mode of antimicrobial action of essential oil of Melaleuca alternifola (tea tree oil). Journal of Applied Microbiology 88, 170–175. |
| [6] | Cronin, M.J., D.S. Yohalem, R.F. Harris and J.H. Andrews, 1996. Putative mechanism and dynamics of inhibition of apple scab pathogen Venturiain equalis by compost extracts. Soil Biol. Biochem., 28: 1241–1249 |
| [7] | Cutler, H.G. and S.J. Cutler, 1999.Biological active natural products: Agrochemicals, CRC Press, Boca Raton, USA, p. 299. |
| [8] | Dubey R.K., Kumar J.R. and N.K. Dubey, 2007. Evaluation of Eupatorium cannabinum Linn. oil in enhancement of shelf life of mango fruits from fungal rotting. World J. Microbiol. Biotechnol. 23: 467–473. |
| [9] | Duru, M.E. A. Cakir, S. Kordali, H. Zengin, M. Harnandar and S. Izumi, 2003. Antifungal activities of the leaves of three Pistacia species grown in Turkey, Fitoterapia,74: pp. 170–176. |
| [10] | Farrag R.S, DawZ.YandS.H Abo-Raya, 1989. Influence of some spice essential oils on Aspergillus parasiticus growth and production of aflatoxins in a synthetic medium. J. Food Sci., 54: 74-76. |
| [11] | FengW. and X.Zheng, 2007. Essential oils to control Alternaria alternatain vitro and in vivo. Food Control 18 (2007) 1126–1130. |
| [12] | Hayes, W.J. and Laws, E.R,1991. Handbook of pesticides ToxicologyVol.1, Academic Press, New York, USA, pp. 55–56. |
| [13] | Imelouane, B., A. Elbachiri, M. Ankit, H. Benzeid and K. Khedid, 2009. Physico-chemical compositions and antimicrobial activity of essential oil of Eastern Moroccan Lavandula dentata. Int. J. Agric. Biol., 11: 113–118 |
| [14] | Macias ,F.A., D. Castellano, R.M. Oliva, P. Cross and A. Torres, 1997. Potential use of allelopathic agents as natural agrochemicals, Proceedings of Brighton Crop Protection Conference—Weeds, pp. 33–38 Brighton, UK. |
| [15] | Maqbool, M., A. Ali and P.G. Alderson,2010. Effect of cinnamon oil on incidence of anthracnose disease and postharvest quality of bananas during storage. Int. J. Agric. Biol.,12: 516–520. |
| [16] | Meepagala K.M, Sturtz G. and D.E Wedge,2002.Antifungal constituents of the essential oil fraction of Artemisia drancunculusL. var. dracunculus. J Agric Food Chem 50:6989–6992 |
| [17] | Perrucci, S., Mancianti, F., Ciont, P.L., Flamini, G., Morelli, I. and, G. Macchioni,1994. In vitro antifungal activity of essential oils against some isolates of Microsporumcanis and M. gypseum. Planta Medica 60, 184–187. |
| [18] | Nascimento S.R.C. S.E. Araujo-Neto and O.M.Hafle, 2000. Use of prochloraz, azoxystrobin and sodium bicarbonate for the postharvest control of Colletotrichum gloeosporioides Tommy Alkins. Summa Phytopathologica;26(3):379-82. |
| [19] | Neler, J., W. Wasserman and M.H. Kutner, 1985.Applied linear statistical models. Regr analysis of variannnce and experimental design. 2ndEd. Richard, D. Irwin Inc. Hame wood Illinois. |
| [20] | Nychas G.J.E.,1995. Natural antimicrobials from plants. In: Gould GW of Melaleuca alternifolia (tea tree oil). J. Appl. Microbiol., 88: 170-175. |
| [21] | Palhano, F.L., T.T.B. Vilches, R.B. Santos, M.T.D. Orlando, J.A. Ventura and P.M.B. Fernandes, 2004. Inactivation of Colletotrichum gloeosporioides spores by high hydrostatic pressure combined with citral or lemongrass essential oil. Int. J. Food Microbiol., 95: 61–66 |
| [22] | Pitarokili D, Tzakou O, Loukis, A and C. Harvala, 2003.Volatile metabolite spp. 441-468.Professional, London, pp. 58-89. |
| [23] | Ramezani, H., H.P. Singh, D.R. Batish, R.K. Kohli and J.S. Dargan, 2002. Fungicidal effect of volatile oils from Eucalyptus citriodora and its major constituent citronellal, New Zealand Plant Protection55: pp. 327–330. |
| [24] | Serrano, M. D. Martinez-Romero, S. Castillo, F. Guillen and D. Valero, 2005. The use of the natural antifungal compounds improves the beneficial effect of MAP in sweet cherry storage, Innovative Food Science and Emerging Technologies 6: pp. 115–123. |
| [25] | Sharma, R.C. and Yadav, P.C., 1996 .Effect of fungicides on Penicillium rot and quality of Chilgoza seed. Phytopathology 49, 77–79. |
| [26] | Shelef, A., (1983). Antimicrobial effects of species. J. Food Saf., 6: 29-44. |
| [27] | Singh, R.K. and R.S. Dwivedi, 1987.Effect of oils on Sclerotium rolfsii causing foot-rot barley. Indian Pythopathol. 40:531-533. |
| [28] | Thangavelu, R., Sundararaju, P., Sathiamoorthy, S., 2004. Management of anthracnose disease of banana caused by Colletotric hummusae using plant extracts. J. Hort. Sci. Biotechnol. 79, 664–668. |
| [29] | Tripathi, P. and N.K. Dubey. 2004. Exploitation of natural products as an alternative strategy to control post harvest fungal rotting of fruit and vegetables. Postharvest Biol. Technol., 32(2): 235-245. |
| [30] | Wilson, C.L., Solar, J.M., El Ghaouth, A. and M.E. Wisniewski, 1997.Rapid evaluation of plant extracts and essential oils for antifungal activity against Botrytis cinerea. Plant Dis. 81: 204-210. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML