-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2012; 2(6): 288-293
doi: 10.5923/j.ijaf.20120206.04
An Evaluation of Segregation Distortion in Wide Crosses of Safflower
Mohammad T. Rabbani 1, Aghafakhr Mirlohi 2, Ghodratollah Saeidi 2, Mohammad R. Sabzalian 2
1Dept. of Plant Science, Faculty of Agriculture, Balkh University, Balkh 1701, Afghanistan
2Dept. of Agronomy and Plant Breeding, College of Agriculture, Isfahan University of Technology, Isfahan 84156-83111, Iran
Correspondence to: Mohammad T. Rabbani , Dept. of Plant Science, Faculty of Agriculture, Balkh University, Balkh 1701, Afghanistan.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Segregation distortion (SD) which is defined as a significant deviation of the observed genotypic frequencies of a locus from the expected Mendelian ratios is a common phenomenon in most mapping studies. This study was conducted to evaluate the occurrence, of the nature and level of the segregation distortion in three wide crosses of Carthamus tinctorius L. and C. oxyacanthus Bieb. using ISSR fingerprinting. Like other interspecific crosses, and since these two species have characters which could be capable of distorting segregation, we expected the segregation distortion could possibly occur. Our results showed that only one population (C4110 × Isf2) deviated from the expected ratio of 1:1 and the other two populations (Isf2 × C4110 and C111 × Isf2) exhibited no distortion. Moreover, the heterogeneity chi square test over the populations indicated that the populations were homogeneous and showed no distortion. It seems that the observed distortion in population C4110 × Isf2 may be due to both genotyping errors and self-incompatibility and, other expected distorting factors could not induce any deviations. This proportion of distortion is the lowest range of distortion in interspecific crosses seen yet. Surprisingly, cyto-nuclear interactions could significantly increase the inherited maternal-specific markers. In addition, our results confirm that the two species of C. tinctorius and C. oxyacanthus have strongly close relationships and interspecific hybridization between two species could directly be exploited in broadening the genetic base of safflower and improving the crop for biotic and environmental abiotic stresses. Also, our result suggests that ISSR molecular markers can be an effective and promising marker system allowing for the establishment of a linkage map along with other molecular markers.
Keywords: Carthamus Tinctorius, C. Oxyacanthus, Segregation Distortion, Heterogeneity Chi Square, Interspecific Hybridization
Cite this paper: Mohammad T. Rabbani , Aghafakhr Mirlohi , Ghodratollah Saeidi , Mohammad R. Sabzalian , "An Evaluation of Segregation Distortion in Wide Crosses of Safflower", International Journal of Agriculture and Forestry, Vol. 2 No. 6, 2012, pp. 288-293. doi: 10.5923/j.ijaf.20120206.04.
Article Outline
1. Introduction
- Mendel’s law of segregation needs prerequisite conditions, namely fair segregation and fair syngamy, to result in the equal transmission of each allele at each locus in each generation. Any factor violating these conditions would lead to segregation distortion (SD) which is defined as a deviation of the observed genotypic frequencies from their expected values[1]. This event is a common phenomenon in most genetic mapping studies. However, these deviations may also have significant impact on the evolution of the genetic structure of populations, because alleles favored by segregation distortion may have tendency to spread throughout a population[2]. Apart from complexes causing segregation distortion to occur, the genetically-controlling mechanisms leading to segregation distortions are poorly understood. Nevertheless, more attention has been attributed to the effects of specific loci in specific regions impacting either gamete transmission or zygote viability[1]. Safflower, Carthamus tinctorius L. (2n = 2x = 24), one of the world’s oldest oilseed crop, has recently attracted considerable attention as an important oilseed crop for arid and semi arid cropping systems[3]. Like major crops, producibility limitations for safflower could be overcome by broadening the genetic base of breeding materials employing interspecific hybridization[4]. The wild species of C. oxyacanthus Bieb. having high naturally crossability[5] and desirable agronomic traits and comparable genetic diversity to cultivated species[6] could possibly be exploited to broaden the genetic base of safflower through interspecific hybridization. Such populations, due to maximum polymorphism, could be employed to construct genetic linkage maps serving as the basis for the development of a saturated map that can be used for localizing, isolating and cloning genes of interest. But, more SD is an unavoidable event in these populations than intraspecific ones[2]. Although, SD has a negative effect on linkage map precision due to biased estimation of the linkage marker distance, they have been used in breeding programs to identify transgenes linked to the pollen gene of the gametophytic self-incompatibility locus in Petunia hybrida[7], and as an instrument to maintain the desired trait in tomato[8]. It is important to have a good knowledge of the occurrence, nature, and level of SD to manage breeding programs and to evaluate which genes will possibly be held together or segregate by SD. As we were involved in both direct and reciprocal types of interspecific crosses in safflower, it was a logical step to examine potential departures from Mendelian expectation. In this research, as the first step for constructing an interspecific linkage map of safflower, we evaluated the level of segregation distortion in three interspecific crosses of C. tinctorius and C. oxyacanthus using Inter simple sequence repeats (ISSR)-polymorphism. ISSR is a PCR-based molecular marker in which 16-25 bp long microsatellites are primed in a single primer PCR reaction to amplify the inter- microsatellite regions[9].
2. Materials and Methods
2.1. Plant Materials
- Two spineless, white seeded and red-flowered genotypes of cultivated safflower, C4110 and C111, and a spiny, yellow-flowered and brown-seeded accession of C. oxyacanthus, herein named Isf2, were used as parents in three crosses,[C4110 × Isf2],[Isf2 × C4110] and[C111 × Isf2]. One plant from each genotype was used as both seed and pollen parents, respectively. The C. tinctorius seeds were selected from Couse land-races and the C. oxyacanthus seeds were from natural habitat of central Isfahan, Iran. Five true interspecific F1 hybrids from each cross detected on the basis of morphological characters[10],[11] and ISSR fingerprinting (data not shown), and, because of self-incompatibility (SI), were inter-crossed to produce F2 populations with totally 130 offsprings. Young leaves of parents, F1 hybrids and F2 progenies were sampled after 4 weeks and stored frozen (-20ºC) until DNA extraction.
2.2. ISSR Analysis
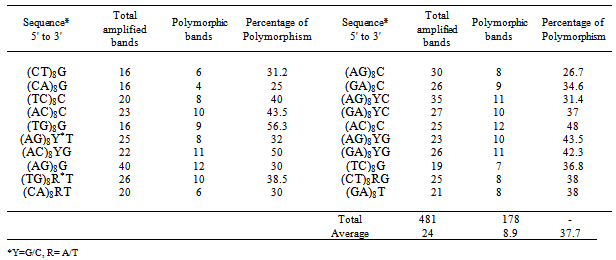
- Total genomic DNA was extracted from fully expanded young leaves according to the Cetyltrimethylammonium Bromide (CTAB) procedure described by Murray and Thompson[12]. A set of 20 ISSR primers capable of producing polymorphic and repeatable fragments[6],[13] were used to amplify inter- SSR sequences of different sizes (Table 1). All of 20 primers were anchored at 3' end with 1 to 3 selective nucleotides differing from the repeat motif were used. Single primer-PCR were carried out in 30 ng of genomic DNA (2 µl), 2 µl of primer (10 pM concentration), 1.5 µl of 10X PCR buffer (500 mM KCl, 100 mM Tris-HCl; pH 8.3), 2 mM MgCl2, 200 μM dNTPs, 1unit of Taq polymerase and 2% formamide for improvement of band resolutions per a total volume of 15µl. The mixture was overlaid with 15 µl mineral oil. PCR amplification was performed in a Techne cycler TC-512 (Techne, UK), programmed for a 2 min initial denaturation at 94°C, followed by 40 cycles of 1 min at 94℃, 1 min at each primer specific-annealing temperature and 2 min at 72℃, and a 7 min final extension at 72℃. PCR products were mixed with loading buffer (98% formamide, 10 mM EDTA, 0.005% of each bromophenol blue and xylene cyanol), denatured at 94°C for 2 min and separated on 6% polyacrylamide gels denatured with 7 mol/l urea at 55℃ at 250 V and 30 W, for 2.5 h and visualized with silver staining protocol[14].
2.3. Data Analysis
- The presence or absence of parental specific markers were scored for parents first and then followed into offsprings from the gels. In order to identify parental polymorphic fragments, a first screening step was performed on the parental genotypes and replicated two times for each primer, and only those fragments that proved to be efficiently amplified each time were subsequently considered on a presence/absence alternative on the progenies. Segregation analysis, due to dominant inheritance of ISSR markers, was checked against the expected 1: 1 theoretical Mendelian ratio (F2 population) using a Chi square analysis (x2=3.841; df= 1; P <0.005), and also homogeneity of populations were determined using chi-square tests for heterogeneity (x2 het= 5.99; df= 2; P <0.005).
3. Results
3.1. Segregation Study
- A total of 481 bands were scored for 20 ISSR primers (Table 1), with an average of 24 bands per primer. Of these amplified bands, 178 bands (37.7%) were polymorphic between parents, averaging 8.9 polymorphic bands per primer. An average of 101 and 92 ISSR markers were specific for cultivated and wild parents, respectively (data not shown). In the crosses of C4110 × Isf2, Isf2 × C4110, and C111 × Isf2, of 178 scored parental specific markers, an average of 113.3, 128.3 and 124 bands were scored in F2 progenies with an average of 121.87 markers, respectively. Overall, 10% of the markers (19.2 markers) scored in the progeny of C4110 × Isf2 did not fit the expected ratio at α = 0.05. Additionally, no segregation distortion towards interspecific cross of neither Isf2 × C4110 nor C111 × Isf2 could be observed (Table 2). Heterogeneity tests revealed no tendency to differ between populations. The data from each F2 populations were homogeneous and could be pooled. The segregation ratio of the pooled F2 data (130 individuals) showed no significant deviation from the proposed 1:1 ratio. Moreover, none of the loci showed uniparental inheritance. This confirmed that amplified fragments were generated from neither chloroplastic nor mitochondrial genome. Also, the heterozygousity of F1 plants confirmed by the simultaneous presence of both loci at each allele in the F2 populations.
|
|
4. Discussion
- Segregation distortion was observed only in 10% of amplified loci in one population and no distortion over the other two populations either direct or reciprocal, is a positive point in an interspecific cross. While, it is reported that the mean value of distortions in interspecific crosses is higher than that of intraspecific ones, 28.7% ± 17.7 for interspecific crosses in comparison to 18.4% ± 11.0 for intraspecific ones[2]. This proportion of distortion has the lowest ranges of distortion in interspecific crosses so far reported. Events which could lead to SD can be started in different developmental stages from sporogenesis to seed germination [15] and also, it could be a result of dysfunctional gametes in pollen, megaspores or both[1]. Despite expecting the segregation distortion, similar to other interspecific crosses, it did not occur. But, we now discuss the probable distorting mechanisms in safflower which might induce distortion.
4.1. Marker Type and Segregation Distortion
- Since marker type has been reported which could lead to segregation distortion, our results showed that, at least in this research, marker type has not led to segregation distortion. ISSR fragments were assumed to descend from multiple genomic loci and to be segregated as dominant markers following simple Mendelian inheritance[16]. Also, agarose gel electrophoresis profiles sometimes present minor bands whose amplifications are capable of variation. For RAPD analysis, it is emphasized that these fragments always demonstrate a lower reproducibility and could remain consequently hazardous to interpret[2]. So, we took use of denaturing polyacrylamide gel electrophoresis to obtain a high resolution gel electrophoresis profile and avoid from co-migration of two bands, and, also we attempted to only score fragments that proved to be efficiently amplified and exhibited clear polymorphism. Nevertheless, the primers used in this study were presumed to be capable of producing clear and repeatable fragments. Because, 3'-anchored end is critical for selective amplification, it is expected that such primers could be prevented from higher propensity for irreproducibility and then segregation distortion. It is reported that the strong selection on marker type would lead to showing no segregation in mapping populations[17]. When such precautions are taken, predominantly ISSR could not induce any distortion. In addition, the same statuses have been reported for RAPD markers[2],[17]. We observed a relatively high rate of inherited (68.45%, data not shown) and polymorphic markers (37.7%, see table 1) over the populations. This amount of polymorphism is comparable to polymorphism observed in interspecific mapping populations derived from other wide crosses of safflower[18]. The ISSR markers take advantages of the SSRs, such as ubiquitous occurrence and high copy numbers in mammalian and plant genomes[19],[20]. While, these markers have a dominant inheritance, it was shown to behave similarly to the co-dominant marker of RFLPs[21] and even in mapping studies, it falls into the regions mapped by the RFLP markers[19]. Despite the advantages of SSR markers and substantial expectation to show no distortions, even, the occurrence of distortions have been reported for SSRs markers, but, much lower than other kinds of markers such as dominant markers of RAPDs and AFLPs[22]. Although, ISSR markers are unmapped but could be exploited to saturate constructed linkage maps, but, in a few studies ISSR markers have been used to map on plant chromosomes [19],[23]. These results could strongly support the widespread distribution of SSRs in the safflower genome. So, the ISSR molecular markers could be an effective and promising marker system allowing for the establishment of a linkage map along with other molecular markers as RFLP, SSR and AFLP.
4.2. Plant Materials and Segregation Distortion
- The parental species used in the present study have different genome sizes[24]. We assumed that attention might be attributed to this genome size variation as mainly the segregation distorter factor. But our results showed that it could not be a segregation distorter in safflower. This character only resulted in more parental specific markers in cultivated genotype compared to wild parents (data not shown). Selfish genetic elements (SGEs) such as transposons making up a large part of the genome of many eukaryotic species[25] could produce ectopically paired chromosomes [26] and lead to segregation distortions by generating abnormal chromosomes after recombinatio n[26],[27]. When two significantly different genomes are brought together in a hybrid, the release, amplification and/or redistribution of retroelements may be promoted by the overall genetic background and cause genome rearrangement, notably on the smallest genome[2],[28]. In two intraspecific crosses of Medicago truncatula and M. tornata, in which the highest proportion of distortion was reported, notably for intraspecific crosses, is correlated with the measured genome size difference[2]. Notice, however, the measurement of 2C nuclear DNA content has been assayed on different genotypes which are endemic to other regions[24]. This could be an exciting task that DNA measurement would be assayed on the same parental genotypes and then subsequent studies analyze the segregation of DNA amounts. In addition, the wild parental genotypes showed self-incompatibility, probably sprophytic system, which forced to intercross the hybrids for producing F2 offsprings. The self-incompatibility either sporophytic or gametophytic has been reported to distort segregation[29]. In the present study, this character was observed more among most of F1 hybrids, notably those of C4110 × Isf2. There may be more self-incompatibility in this population, besides the genotyping error, is one of the segregation distorter in this research. A sprophytic self-incompatibility system with six alleles has described in wild relative of cultivated safflower, C. flavescens Spreng[11]. The low level of deviation in intraspecific crosses is often attributed to a close genetic proximity between the parents[2]. In contrast, the high number of distorted loci in the interspecific population may have been due to recombination suppression at meiosis caused by a considerable degree of non-/or partial-homology between the chromosomes[30]. The two species of C. tinctorius, and C. oxyacanthus have considered as separate species, but they are more likely races of a biological species[10],[11]. It seems that, however, the parental genotypes used in this research were from two species, but they have relatively close relationship [5],[6],[31],[32]. Because they are endemic to the same habitat and gene introgression is a probable event which might be occurred between species for a while[6]. The wild species is completely self-incompatible providing a suitable manner to cross-pollination occurs. This event could change the genetic structure and create a partially similar genetic background. So, even an interspecific hybridization would establish, due to high background similarity, the occurrence of distortion will be less, as we observed in our investigation. Recently, it has been reported that the intraspecific mapping populations of safflower have showed a higher degree of distorted loci than the interspecific populations[18]. However, the fortunate choice of parents has a considerable impact on the rate of polymorphism, we established this study with the safflower accessions (C111, C4110 and Isf2) that previously analyzed for genetic diversity and phylogenetic relationships, and, they had showed high genetic and agro-morphological variation[6]. Therefore, we assumed that the rate of polymorphism would be higher than we expected. Mayerhofer et al.[6] found of 8% polymorphism in their research, compared with 37.7% polymorphism in our study. This large amount of difference is probably due to fortunate choice of plant materials and primers set. We observed polymorphism rate of 37.7% in our study with the primers previously showing an average polymorphism rate of 93.41%[6]. For instance, Sabzalian et al.[6] reported that the primers (AC)8G and (AC)8YG have polymorphism rates of 95% and 85%, respectively. Using the same primers, we found polymorphism rates of 43.5% and 50%, respectively. Noticeable, onto the recently published linkage map for safflower, it is reported that the SSR primers used in this study showed a relatively low degree of polymorphism in C. tinctorius and C. oxyacanthus (8% in compared to 11.2%), respectively. In addition, most of them have showed a uniparental polymorphism which is a negative point for this type of markers that could not clearly uncover the genetic variations at molecular level. Also, relatively the same rate of polymorphism for SSR markers (6.9%) have been reported in an interspecific map of in C. tinctorius and in C. palaestinus[33]. Also, maternal-specific markers were significantly affected by cross direction. In the cross of Isf2×C4110, in compared to C4110× Isf2, changing the cross direction caused up to 6.5% increase in maternal-specific markers. It could be due to effects of genetic-cytoplasmic (cyto-nuclear) factors as chloroplast and mitochondria on cell activities like meiosis, crossing over and chromosome transmition. On other hand cross direction could significantly influence the rate of inherited markers from parents into offsprings. The effects of maternal factors have been reported in alloplasmic lines of barley [34] and hybrid structure in sunflower[35]. These lines of evidences clearly support that the two species of C. tinctorius and C. oxyacanthus have strongly close relationship and agree with previously reports utilizing data from cytogenetics and DNA markers [4],[6],[31],[32]. In addition, our results along with a published research[18] show the applicability of interspecific hybridization between the two species of C. tinctorius and C. oxyacanthus, due to relatively equal transmission of characteristics from parents to offsprings and thus less expected distortions.
5. Conclusions
- The results of this study showed that the two species of C. tinctorius and C. oxyacanthus have very close genetic relationship, and, are more likely races of a biological species. And due to relatively equal transmission of characteristics from parents to next generations, interspecific hybridization between these two species could be an effective manner to broaden the genetic base of safflower gene pool. But, it is considerable that the parental materials and cross direction are assumed to be factors influencing genetic structure of resulting populations. Also, we believe that the data from ISSR markers strongly support the widespread distribution of SSRs in the safflower genome. So, ISSR markers can be a promising marker system allowing for the establishment of a linkage map along with other molecular markers. Further studies with either intra- or interspecific crosses, larger populations, and more molecular markers would be helpful in investigating the occurrence and potential reasons for segregation distortion in safflower.
ACKNOWLEDGEMENTS
- We greatly appreciate Dr. Denis Eaton for reading an early version of the manuscript and acknowledge Isfahan University of Technology for financially supporting this research.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML