-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2012; 2(6): 279-287
doi: 10.5923/j.ijaf.20120206.03
The Host Range of Phomopsis cirsii; a Potential Biological Control Agent of Cirsium Arvense
Vibeke Leth , Christian Andreasen
Department of Agriculture and Ecology, Faculty of Science,University of Copenhagen, HoejbakkegaardAllé 13, DK-2630 Taastrup, Denmark
Correspondence to: Christian Andreasen , Department of Agriculture and Ecology, Faculty of Science,University of Copenhagen, HoejbakkegaardAllé 13, DK-2630 Taastrup, Denmark.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
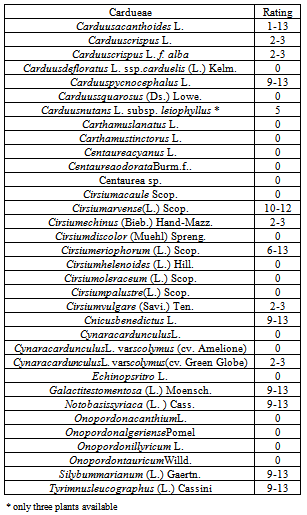
Cirsiumarvense is a noxious perennial weed which has become an increasing problem in North European countries partly because of restriction in use of effective herbicides.Mechanical weedingislabour intensive and expensive and therefore there is a need for an additional method likebiological control. An isolate PKDK101 of the fungus Phomopsiscirsii, which is virulent to C. arvense causing stem canker and die back was chosen to test the specificity of the fungus. A series of infection trials were successively carried out on 127 plant species (incl. ssp. and var.) belonging to 16 families in greenhouses in order to encircle the host range of P. cirsii. Susceptible plant species were found only in the thistle group (Cardueae) which contained 34 species belonging to 12 genera. Susceptible species were found in thirteen of these genera. Highly susceptible species included Carduusacanthoides, Carduuspycnocephalus, Cirsiumeriophorum, Cnicusbenedictus, Galactitestomentosa, Notobasissyriaca, Silybummarianum and Tyrimnusleucographus, which showed symptoms from girdling of stem, heart rot in rosettes to death of entire plants. Mild and restricted symptoms were observed on Carduuscrispus, Carduusnutans, Cirsium echinus, Cirsiumvulgare and Cynaracardunculusvar.scolymus (artichoke) with symptoms such as restricted necrotic leaf spots and too early senescence or death of entire leaf. Eleven hosts for P. cirsii were recorded but despite the expanded range of hosts we expect that its host range will be within Cardueae.P.cirsii,poses multi-target potential against several annual and biennial weedy thistles from warmer climates. The pathogenicity of P. cirsii towards the artichoke, however, could limit its field of application especially in the Mediterranean area. The potential of P. cirsii as a control agent, in areas where artichokes are cultivated, would depend on the existence of P.cirsii resistant varieties or the existence of P.cirsiiisolates non-pathogenic to artichoke.
Keywords: BiologicalControl, Canada Thistle, Phomopsiscirsii, Host Range, Multi-Target Potential, Mycoherbicide
Cite this paper: Vibeke Leth , Christian Andreasen , "The Host Range of Phomopsis cirsii; a Potential Biological Control Agent of Cirsium Arvense", International Journal of Agriculture and Forestry, Vol. 2 No. 6, 2012, pp. 279-287. doi: 10.5923/j.ijaf.20120206.03.
Article Outline
1. Introduction
- Cirsiumarvense (L.)Scop.is one of the world’s most troublesome and persistent perennial weeds[1],[2]. In dense stands crop loss can exceed 70%[3]. Contamination of seed, grain or crop straw reduces quality, and spines are a source of physical damage to animals. C. arvense has become an increasing problem in North European countries especially in organic agriculture[4],[5],[6],[7],[8]. The plant produces an extensive far-creeping and deep root system, which insures survival and rapid vegetative spread. New aerial shoots can arise at any point along the horizontal root resulting in dense patchesonly a few years after infestation [2],[9]. Long distance dispersal of the plant happens from pieces of roots as well as seeds [10].Restrictions in use of effective herbicides (e.g.phenoxy-herbicides), the increasing area with organicagriculture and the widespread establishment ofset-asideduring the 90’ties and the beginning of this century are possibly responsible for the increasing abundance of C. arvense on arable land in the Nordic countries[4],[5], [11],[12],[13].In organic cropping systems repeated cultivation or cutting are used to starve the roots and prevent further shoot emergence and assimilation [11],[14]. Such treatments are labour intensive, expensive, and require the right equipment which many farmers do not have. Hence, there seems to be need for alternative or additional control methods in arable cropping systems.Several pathogens with potential as biological control agents have been studied such as Sclerotiniasclerotiorum (Lib.) de Bary(e.g.[15],[16]), Alternariacirsinoxia Simmons & Mortensen[18],[19], Pucciniapunctiformis (Str.) Röhl. (e.g.[20]) and PhomadestructivaPlowr.[21], but none of these pathogens have been developed into effective mycoherbicides against C. arvense.Phomopsiscirsii Grove is commonly found on diseased C. arvense in Denmark[22] and representative isolates of this fungus have been verified by Dr. E. Punithalingam at the International Mycological Institute (IMI), and isolates were deposited (IMI no. 287751 and 278416) for patent purpose [23]. Findings have also been recorded on this host from Norway[24] and England[25]. Adding to these findings, P.cirsii has been recorded from Cirsiumpalustre L. (Scop.) in Norway[26], Cirsiumeriophorum L. (Scop.) in England[24] and more recently on Cirsiumvulgare (Savi.) Ten.in Germany[27]. Its virulence and aggressiveness towards C. arvense has been proven in glasshouse trials. An isolate (PKDK101) were able to kill all infested C. arvense plants within 21 days. The first symptoms appeared 5–7 days after inoculation, typically as dark brown or black spots or stripes on the leaf veins, most frequently on the young leaves or the stem. The fungus invaded the stems directlyor most frequently via the leaf veins and after girdling of the stem, it always grew downwards towards the roots, causing gradual die back of the shoots[22]. Approximately 65 of the species of Phomopsis listed by Uecker[28] are considered to be plant pathogenic and host specific. So far, at least four species have been investigated for potential as bioherbicide agents. Shivas et al.[29] demonstrated that P. emecisShivas, the causal orgasms of stem blight of the noxious weed EmexaustralisStein., was pathogenic to five closely related species in the Polygonaceae, and that inoculation of other unrelated plant species resulted in infection only when the plants were wounded or were senescent, and that the organism did not advance to the healthy tissues.The fungus Phomopsisamaranthicola Rosskopf, Charudattan, Shabana, & Benny targeting Amaranthus spp. has been patented for Amaranthus control[30, 31]. Host range testing has been performed on 21 species in the genus Amaranthus and 56 plant species outside the genus Amaranthus, including crops, and members of genera that are closely related to Amaranthus. P.amaranthicola did not infect any of the plants from outside the genus Amaranthus but was highly pathogenic to several of the species in the genus Amaranthus[32]. The pathogen has shown varying efficacy. Despite plants being given an initial dew period, P. amaranthicola did not cause mortality on any Amaranthus species in greenhouse or under field conditions in experiments conducted in south Texas [33]. A large number of isolates of Phomopsissp. has been collected from the weedCarthamuslanatus L. (saffron thistle) in Australia, and their potential as biological control agents against weeds of the Asteraceae has been demonstrated[34].The susceptibility of Convolvulus arvensis L. accessions from different geographic locations to disease caused by the fungal pathogen, Phomopsis convolvulusOrmeno, has been evaluated[35]. The emerging shoots of accessions showed severe disease development and the fungal application on Greek and Montana accessions reduced aboveground biomass 83 to 100% and 65 to 86%, respectively. Results of this study indicate that control of C. arvensis using P. convolvulus might be achieved in various geographic regions[35]. Conclusively, Phomopsis spp. may be candidates as bioherbicides for several weed species.The objective of this study was to determine the host range of P.cirsiisince biological control agents should be environmentally safe and unwanted side-effects on the wild flora, crops and ornamental plants should be avoided.
2. Material and Methods
- A series of experiments were carried out in order to define the host range of the fungus.The host range was evaluated qualitatively in greenhouses on a selection of available crop, ornamentals and wild plant species tested according to the centrifugal phyllogenetic scheme suggested by Whapshere [36].
2.1. Plant Material
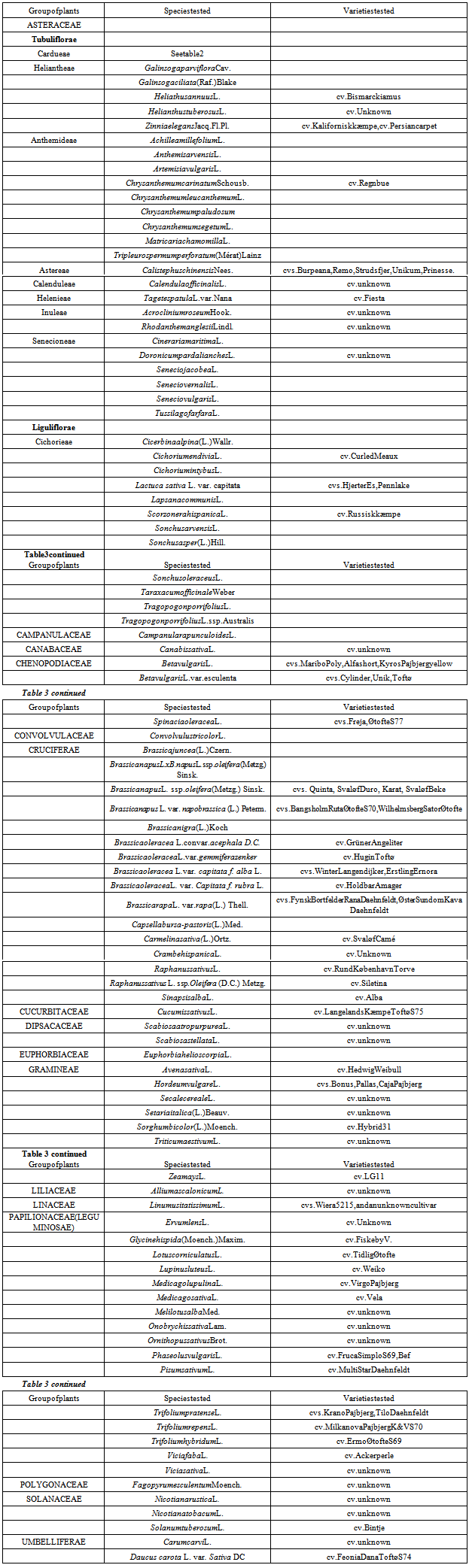
- A range of test plants belonging to 108 species and 37 genera were propagated from seeds, tubers or roots. Seeds of plants exotic to Denmark were either provided by the Botanic Garden, University of Copenhagen or bought at seed stores. Seeds of endemic wild plants were collected locally and crop plants grown in Denmark were provided by the Faculty of Sciences, University of Copenhagen or from local seed stores. Healthy looking seeds from the dicotyledonous plants were sown in trays in a 1:3 mixture of gravel and peat soil (Pindstrup no. 2, pH 5.6-6.6) and healthy looking seedlings at the two true leaf stage were transplanted into 13 cm diameter plastic pots. Plants of C. arvense were cloned from 3-5 cm long root pieces with at least two root buds. Monocot plants were established in 13 cm diameter pots containing 10 seed per pot and grown without transplanting. The plants were grown under greenhouse conditions with supplemental lighting 12 hours day-1, supplied by 400 Watt Phillips mercury lamps. Day and night temperatures fluctuated between 13 and 33℃ with means of 16-20℃ and 20-25℃ respectively. Pests were controlled using yellow sticky traps. The plants were watered individually according to requirement.
2.2. Inoculum Production
- The fungus P.cirsii isolate PKDK101[22] was used in all 10 trials. The fungus was cultivated in Roux glass bottles on the surface of 250 ml of sterile CzapeckDox Broth with 0.01 % DifoBacto agar (DifcoMicrobiology). The bottles were inoculated with four plugs (4 cm2) cut from actively growing margins of colonies (fig. 1B) on Potato Dextrose Agar(PDA) and incubated for 4-5 weeks at 20-25°C in diffuse light in the laboratory. The resulting mycelial mats were then harvested and prepared for inoculation as described by Lethet al.[22]. The final inoculum consisting of a suspension of mycelial fragments was adjusted with sterile deionised water to contain 80 g of mycelium per litre (0.08 g ml-1).
2.3. Inoculation of Plants
- The virulence and aggressiveness of P.cirsii isolate PKDK101 on Cirsiumacaule(L.) Scop.,Cirsiumcarlinoides Fisch. and Carduusthoermeri Weinm. wastested using three to six plants due to unavailability of sufficient numbers of seeds. All other tests were done using 10-15 test plants per species and variety. For cereals five pots were sprayed. As a control, the same numbers of the plants in question were sprayed to run off with deionised water. In order to confirm the pathogenicity of the inoculum, five plants of C. arvense grown from root pieces of a susceptible Danish clone were co-inoculated at each of the ten successive infection trials. The infection trials were carried out one to two weeks apart and for annual plants at the six leaves to flower bud stage; for biennial and perennial plants in the rosette stage. Cereals were tested when they had developed four to six leaves. The plants wereinoculated by spraying to run off with the mycelia suspension, using compressed air (2 kg cm-2) and a spray gun.The inoculated plants were then covered by polyethylene bags and incubated 72 hours under greenhouse conditions before removal of the bags. During the daytime the plants were protected against sunlight using sheets of white paper while incubated in the plastic bags. As quality assurance, an experiment was accepted when at least three out of the five C.arvense control plants showed symptoms of infection 14 days after inoculation (DAI), otherwise the same plants were re-inoculated with a new batch of inoculum.
2.4. Disease Rating
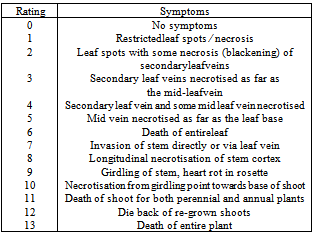
- The inoculated plants were evaluated for disease symptoms 21 DAI according to the previously developed disease severity scale for the P.cirsii- C.arvensepathosystem (Table 1)[22].
|
3. Results
4. Discussion
- No matter how effective the biological control agent is, host specificity remains the crucial filter for the selection of biological control agents. Unwanted side-effects on non-target plant species may have serious consequences for the food-web in the ecosystem and economic consequences for the society. As a consequence of increasing awareness of possible side-effects of biological control, the degree of acceptable risk, tolerated by regulatoryauthorities is becoming less and less, even in countries where biological weed control has been widely accepted and successful (e.g.[37],[38]). According to Whapshere[36] it should be expected that a bio-control agent isrelativelyspecific ifit only attacks some of the plant species closely related to the target plant.However, due to the weak species concept of the form-genus Phomopsisand its importance as pathogens ofmany different crop plants[22],[28], it was decided that the present experiments should include an extended number of plant genera and species.Whapshere´s assumption did hold true for the present P. cirsii isolate which kept its host range within the tribe Cardueae, and even with great variation in susceptibility of the closest related species to C. arvense (Cirsium, Carduus), reaching from resistant (0) to highly susceptible (9-13) Increasing knowledge about the biology of the genus Phomopsis has revealed that some species are endophytes and invaded the host without creating symptoms or resulting in latent infections on some of the apparently resistantspecies of the Cardueae, especially,on the true thistlesCirsium and Carduus spp..However, none of the symptomless plants expressed symptoms during the prolonged incubation period, until senescence occurred. The host preference may vary among isolates of P. cirsii, but can be expected to remain within Cardueae. Previous studies have shown that the cultivation conditions of the fungus may influence its virulence [22]. In the present series of inoculations of test plants the PKDK101 isolate remained virulent to the C. arvense control plants, except for one set of plants, which had to be successfully re-inoculated.
|
5. Conclusions
- The results from this study show that P.cirsii, which to our knowledge hasonly been recorded from thistlesinDenmark, England, Germany and Norway[22],[25],[26],[27],poses multi-target potential against several annual and biennial weedy thistlessuch as Cirsiumarvense,Carduuspycnocephalus L., CnicusbenedictusL., Galacitestomentosa(L.) Moensch, Notobasissyriaca (L.) Cass and Silybummarianum (L) Gaertn..The P. cirsii isolate kept its host range within the tribe Cardueae, and with great variation in susceptibility of the closest related species to C. arvense (Cirsium, Carduus), reaching from resistant (0) to highly susceptible (9-13). The pathogenicity of P. cirsii towards the artichoke, however, could limit its field of application especially in the Mediterranean area. The potential of P. cirsii as a control agent, in areas where artichokes are cultivated, would depend on the existence of P.cirsii resistant varieties or the existence of P.cirsiiisolates non-pathogenic to artichoke. Further studies should include repeated specificity tests with different isolates of P. cirsii concentrating on crop plants from the Cardueaegroup (Cynara spp. and Carthamus spp.) and on endangered thistle species.
ACKNOWLEDGEMENTS
- This work was founded by The Agricultural- and Veterinary Research Council (SJVF) and carried out at the Faculty of Science, University of Copenhagen. The author is grateful to Mr. Niels Henrik Sørensen and to Ms. Yvonne Nyskjold for assistance and to Dr.FolmerArnklit, Botanic Garden of Copenhagen for providing seeds of exotic plants.
References
| [1] | Holm LG, Plucknett DL, Pancho JV and Herberger, JP (eds), Cirsiumarvense (L.) Scop. The World's Worst Weeds. Distribution and Biology, pp. 217–224. University Press of Hawaii, Honolulu, Hawaii, USA. 1977. |
| [2] | Tiley GED. Biological Flora of the British Isles: Cirsiumarvense (L.) Scop. Journal of Ecology, 98, 938-983, 2010. |
| [3] | Behrens R and Elakkad MA, Canada thistle interference in crops. Canada Thistle Symposium. In Proceedings of North Central Weed Conference, 36, 167–169, 1981. |
| [4] | Andreasen C and Stryhn H, Increasing weed flora on Danish arable fields and its importance for biodiversity. Weed Research, 48, 1–9, 2008. |
| [5] | Andreasen C and Stryhn H, Increasing frequency of weed species in Danish beet, pea and winter barley fields. Crop Protection, 36, 11–17,2012. |
| [6] | Rydberg NT and Milberg P, A survey of weeds in organic farming in Sweden. Biological Agriculture and Horticulture, 18, 175–185, 2000. |
| [7] | Salon J, Hyvönen T and Jalli H, Weed flora in organically grown spring cereals in Finland. Agricultural and Horticultural Food Science in Finland, 10, 231–242, 2001. |
| [8] | Verschwele A and Haüsler A, Effect of crop rotation and tillage on infestation of Cirsiumarvensein organic farming systems. In: Proceedings of the 6th EWRS (European Weed Research Society) Workshop on Physical and Cultural Weed Control (eds DC Cloutier& J Ascard), 187–194. Lillehammer, Norway, 156–163. European Weed Research Society, Doorwerth, The Netherlands. 2004. |
| [9] | Bakker D, A comparative life-history study of Cirsiumarvense (L.) Scop. andTussilagofarfara L. the most troublesome weeds in the newly reclaimed polders of the former Zuiderzee. In: Symposium on Biology of Weeds, British Ecological Society (ed JL Harper), Blackwell Scientific Publications, Oxford, UK, 205–222, 1960. |
| [10] | Hettwer U and Gerowitt B, An investigation of genetic variation in Cirsiumarvensefield patches. Weed Research, 44, 289–297, 2004. |
| [11] | Dock Gusstavson A-M, Growth and regenerative capacity of plants of Cirsiumarvense. Weed Research, 37, 229–236, 1997. |
| [12] | Hyvönen T and Huusela-Veistola E, Arable weeds as indicators of agricultural intensity - A case study from Finland. Biological Conservation, 141, 2857–2864, 2008. |
| [13] | Andreasen C and Streibig JC, Evaluation of changes in weed flora in arable fields of1Nordic countries – based on Danish long-term surveys. Weed Research, 51, 214–226, 2011. |
| [14] | Graglia E, Melander B and Jensen RK, Mechanical and cultural strategies to control Cirsiumarvensein organic arable cropping systems. Weed Research, 46, 304–312, 2006. |
| [15] | Brosten BS and Sands DC Field trials ofSclerotiniasclerotiorumto control Canada thistle (Cirsiumarvense). Weed Science, 34, 377–380, 1986. |
| [16] | Bourdôt GW, Harvey IC, Hurrell GA and Saville DJ, Demographic and biomass production consequences of inundative treatment of CirsiumarvenseandSclerotiniasclerotiorum. Biocontrol Science and Technology, 5, 11–25, 1995. |
| [17] | Bourdôt GW, Baird D, Hurrell GA and De Jong MD, Sclerotiniasclerotiorum-based mycoherbicide: accounting for regional and yearly variation in climate. Biocontrol Science and Technology, 16, 345–358, 2006. |
| [18] | Simmons EG and Mortensen K, Alternariathemes and variations. No.218. AlternariacirsinoxiaSimmons and Mortensen, sp. nov.Mycotaxon, 53, 72–76, 1997. |
| [19] | Green S, Bailey KL and Tewari JP, The infection process of Alternariacirsinoxiaon Canada thistle (Cirsiumarvense) and host structural defense responses. Mycological Research, 105, 344–351, 2001. |
| [20] | Frantzen J, An epidemiological study ofPucciniapunctiformis(Str.) Röhl as a stepping-stone to the biological control of Cirsiumarvense(L.) Scop. New Phytologist127, 147–154, 1994. |
| [21] | Kruess A (2002) Indirect interaction between a fungal plant pathogen and a herbivorous beetle of the weed Cirsiumarvense. Oecologia, 130, 563–569, 2002. |
| [22] | Leth V, Netland J and Andreasen C, Phomopsiscirsii: a potential biocontrol agent of Cirsiumarvense. Weed Research 48, 533–541, 2008. |
| [23] | Leth V, Inventor of Patent ‘‘Herbicide Containing phytotoxic fungal material from Phomopsiscirsii or Septoriacirsii, especially for control of Compositae’’. Patent no. EP 13685A, AU 8432760A, NO 8403545A, FI 8403493A, J 60084207A, DK 8404254A, ZA 8407001A, US 4753670A, CAT 247879A, DE 3475745G, EP136850B. 1985. |
| [24] | Netland J (2011) Bioforsk, Norwegian Institute of Agricultural and Environmental Research. E-mail jan.netland@bioforsk.no (personal communication). |
| [25] | Grove WB, British Stem- and Leaf-Fungi (Coelomycetes). – Cambridge University. Cambridge, UK, 1935. |
| [26] | Jørstad I, Septoria and septoroid fungi on dicotyleones in Norway. Oslo University Press, p. 91. 1965. |
| [27] | Ale-Agha N, Feige GB andDachowski M (2002) Microfungi on compositae in the Ruhr Basin. Mededelingen (Rijksuniversiteitte Gent. Fakulteit van de Landbouwkundige en ToegepasteBiologischeWetenschappen), 67, 217-26, 2002. |
| [28] | Uecker FA, A World List of Phomopsis Names with Notes on Nomenclature, Morphology and Biology. Mycologia Memoire No. 13, The New York Botanical Garden. J. Cramer, Berlin. 1988. |
| [29] | Shivas RG; Lewis JC and Groves RH, Distribution in Australia and Host-Plant specificity of Phomopsis-Emicis, a stem blight pathogen of Emex-Australis. Australian Journal of Agricultural Research 45, 1025-1034, 1994. |
| [30] | Charudattan, R, Shabana, YM, DeValerio, JT and Rosskopf EN, A broad-spectrum bioherbicide for controlling pigweed species. U.S. Patent No. 5,393,728. February 8, 1995. |
| [31] | Charudattan, R., Shabana, YM, DeValerio, JT and Rosskopf EN, Phomopsis species fungus useful as a broad-spectrum bioherbicide to control several species of pigweeds. U.S. Patent No. 5,510,316. April 23, 1996. |
| [32] | Rosskopf EN, Yandoc CB and Charudattan R, Genus-specific host range of Phomopsisamaranthicola (Sphaeropsidales), a bioherbicide agent for Amaranthus spp. Biocontrol Science and Technology, 16, 27–35, 2006. |
| [33] | Moran PJ and Showler AT, Phomopsisamaranthicola and Microsphaeropsisamaranthi Symptoms on Amaranthus spp. Under South Texas Conditions. Plant Disease, 91, 1638–1646, 2007. |
| [34] | Ash GJ, Stodart B, Sakuanrungsirikul S, Anschaw E, Crump N, Hailstones D and Harper JDI, Genetic characterization of a novel Phomopsis sp., a putative biocontrol agent for Carthamuslanatus. Mycologia, 102, 54–61, 2010. |
| [35] | Vogelgsang, S, Watson A.K,.DiTommaso A, and Hurle K, Susceptibility of Various Accessions of Convolvulus arvensis to Phomopsis convolvulus. Biological Control, 15, 25–32, 1999. |
| [36] | Whapshere A J, A strategy for evaluating the safety of organisms for biological weed control. Annals Applied Biology, 77, 201–211, 1974. |
| [37] | Sheppard AW, Heard TA and Briese DT,Workshop recommendations: the selection, testing and evaluation of weed biological control agents. In: Improving the Selection, Testing and Evaluation of Weed Biological Control Agents (eds H Spafford-Jacob & DT Briese), pp. 89–100. CRC Technical Series No. 7. CRC for Australian Weed Management, Glen Osmond, Australia, 2003. |
| [38] | McFadyen RE, Biological control: managing risks or strangling progress? In: Sindel BM & Johnson SB (eds). Proceedings of 14th Australian Weeds Conference. Weed Society of New South Wales, Sydney Australia, pp. 78-81, 2004. |
| [39] | Stace C (Ed), New flora of the British isles (2ed edition) Cambridge University Press, Cambridge, UK. 2001. |
| [40] | Tutin TG, Heywood VH, Burges NA, Moore DM, Valentine DH, Walters SM and Webb DA. (eds), Flora Europaea, Vol. 4. Plantaginaceae to Compositae (and Rubiaceae). Cambridge University Press, Cambridge, 1976. |
| [41] | Galán E, Prados F, Pino A, Tejada L and Fernández-Salguero J, Influence of different amounts of vegetable coagulant from cardoon Cynaracardunculus and calf rennet on the proteolysis and sensory characteristics of cheeses made with sheep milk. International Dairy Journal, 18, 93–98, 2008. |
| [42] | Grammelis P, Malliopoulou A, Basinas P and Danalatos NG, Cultivation and characterization of Cynaracarduculus for solid biofuels production in the Mediterranean region. International Journal of Molecular Science, 9, 1241 –1258, 2008. |
| [43] | Holm L, Pancho JV, Herberger JP and Plucknett DL, A geographical atlas of world weeds. John Wiley & Sons, NY, Chichester, Brisbane, Toronto. 1979. |
| [44] | Bianco VV, Present situation and future potential of artichoke in the Mediterranean basin. ActaHorticulturae 681, 39–55, 2005. |
| [45] | Ryder EJ, De Vos NE and Bari MA, The globe artichoke (Cynarascolymus L.). HortScience 18, 646–653, 1983. |
| [46] | Sonnante G, Pignone D and Hammer K, The domestication of artichoke and cardoon: from Roman times to the genomic age. Annals of Botany, 100, 1095–1100, 2007. |
| [47] | Hahn GG, Life history studies of species of Phomopsis occurring on conifers. Transaction of the British Mycological Society, 15, 32–93, 1930. |
| [48] | Grove WB, British Stem- and Leaf-Fungi. Vol. 2. Cambridge, England. Cambridge University Press. 1937. |
| [49] | Haye T, Goulet H, Mason PG and Kuhlmann U, Does fundamental host range match ecological host range? A retrospective case study of a Lygus plant bug parasitoid. Biological Control, 35, 55–67, 2005. |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML