-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2012; 2(5): 225-234
doi: 10.5923/j.ijaf.20120205.05
Mechanical Impedance Effects on Growth, Phenolics and 2,4-dihydroxy-7-methoxy-2H-1 ,4-benzoxazin-3-one (DIMBOA) in Root Exudates of Zea mays L
Alberto de J. Oliveros-Bastidas 1, Francisco A. Macías 2, J. M. G Molinillo 2
1Laboratorio de Química Ecológica, Departamento de Química. Facultad de Ciencias. Universidad de Los Andes. Núcleo Universitario Pedro Rincón Gutiérrez. La Hechicera. Mérida, 5101, Estado Mérida. Venezuela
2Grupo de Alelopatía, Departamento de Química Orgánica, Facultad de Ciencias, Universidad de Cádiz, C/. República Saharaui s/n, Apdo. 40, 11510, Puerto Real, Cádiz, Spain
Correspondence to: Alberto de J. Oliveros-Bastidas , Laboratorio de Química Ecológica, Departamento de Química. Facultad de Ciencias. Universidad de Los Andes. Núcleo Universitario Pedro Rincón Gutiérrez. La Hechicera. Mérida, 5101, Estado Mérida. Venezuela.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The purpose of this work was to evaluate the direct effect of mechanical impedance (IM) on root exudation from the indirect effect involving root morphological modifications induced by IM. Zea mays L. plants were grown in axenic hydroponic culture conditions for 4, 8, 12 and 16 days, and mechanical impedance was simulated by glass beads. At the end of the culture, exudation of 2,4-dihydroxy-7-methoxy-2H-1,4-benzoxazin-3-one (DIMBOA) for plants in a nutrient solution was measured. At harvest, plant growth and development parameters as well as DIMBOA and phenolics total in root exudate were measured. The results demonstrated a major influence of mechanical impedance on root growth with a reduction in shoot elongation. A medium which shows a higher resistance to growth, resulting in increased levels of DIMBOA y phenolic derivate. For high and low IM to 12 days of growth are obtained 6.94±0.36 and 5.47±0.37 µg DIMBOAxmL-1xseedling (n=3), showing significant differences (ANOVA, p <0.001); although the effect is time dependent growth. Correlations between plant morphology and root exudation suggest that root morphology is probably involved in the modification of root exudation. The results indicate that the resistance provided by growth medium will have a significant effect on the chemistry of the plant, and consequently on the ecology. This is important especially in crop species, where a new variable arises in crop management.
Keywords: Allelochemicals, Benzoxazinoids, Bioassay, Exudates root, DIMBOA, HPLC, Mechanical Impedance, Zea mays L
Article Outline
1. Introduction
- The soil environment in a natural ecosystem is heterogeneous both in time and space, even in micro-scale[1], and plant response to these heterogeneous environments may include changes in biomass accumulation, speed of nutrient uptake or root morphology[2-7]. From the point of view of morphology, these have been described as the most significant changes in soil heterogeneity occurring in the roots[8], and it is known that factors such as soil compaction, have a profound effect on development root, and as a result of these, the whole plant[9-15]. Due to the importance of these effects on plants there have been economic interest, many studies have been conducted in intensive cultivation of plants, and some results, such as a decrease in plant growth, decreased root access of water and nutrients, are consequences of this effect, and hence the importance generated in crop management[16-22].From the morphological point of view, the effect of soil resistance to penetration and root growth (mechanical impedance-IM), demonstrates a decrease in root growth, increased stem diameter and an increase in the root diameter disabled[23, 24].There are several studies on these and other morphological characteristics, no clutch, from a physiological standpoint, the study on the effect of IM is less well documented, especially about the effect this has on the phenomena of exudation and its possible consequences for the ecology of the plant. Most recent studies analysing the effect of IM on the levels of exudates have been carried out on seedlings of corn (Zea mays L.) in a hydroponic growth medium[25-37]. In these studies we have simulated the effect of IM from the growth medium using glass beads, which showed a correlation between morphological features of the seedling and total carbon levels in the exudates. Given that the root exists a direct relationship between morphology and physiological processes in it are carried out, as is the phenomenon of bleeding, it should not surprising to find significant differences in chemical content, both qualitatively and quantitatively, their exudates over IM.It seems to be a distinct accumulation and exudation by roots group for maize seedling, suggesting that any change in the architecture of the root, may have important consequences on their ability to exude defence compounds, measured here as content of 2.4-dihydroxy-2H-benzoxazin- 3-one (DIMBOA). For maize (Z. mays L.), it has been found that the degree of mechanical impedance or soil compaction has important effects on the density of the different groups of roots[38], especially in elongation seminal roots, where there has been a decrease in length with increasing soil density.With this background it is logical to hypothesize that the IM can cause changes in the levels of chemical compounds with allelopathic characteristics, such as phenolic acids and benzoxazinoids[39,40], and some result variations registered in the organic compounds soluble in water according to the gradient of IM, support this hypothesis[28,41-43].Recent studies on the effect of IM on corn seedlings and chemical composition in the presence of an effect on the development of the whole plant, significantly affecting the content of compounds in exudates, these last one measured as carbon total[28,44], conclude that the morphology is a factor that governs the process of exudation. To date there are no data on the influence of the resistance of the culture medium in the exudation of defence compounds, specifically in plants with the capacity to exude allelopathic compounds with potential as the DIMBOA in the case of maize, where many of the interactions and defence mechanisms proposed around this plant are related to production levels of these defence compounds by root exudates.From a practical standpoint, raising awareness about the effect of soil conditions on the levels of defence compounds that can be transmitted to the rhizosphere, will undoubtedly be of great importance in the study of cultural practices on crops with potential allelopathic, and management practices in crop rotation and cover.On the other hand, from a methodological standpoint, it is important to know how it can affect bioassay conditions by which to measure the allelopathic potential of many species, usually found as many designs to measure the allelopathic potential, and the great Most use purely hydroponic media[40,45-53], without considering the effect of MI, thus, support crop or IM grade must be considered an indicator of degree of allelopathic activity observed.With these ideas, in this paper we study the effect of culture medium (agar and glass beads), not on total exudates, but the levels in exudates of allelopathic compounds with potential as DIMBOA and phenolic derivatives. Moreover, less information is available on the effect of mechanical impedance on root exudation taking into account the influence of plant age. Previous studies on the influence of MI in the root exudates of maize have shown that the amount of organic material total released by growing roots decreases with time[41, 54]. The effect of IM, on the kinetics of exudation of DIMBOA and phenolic acid total, was therefore investigated for plants from 2 to 16 days old.
2. Material and Methods
- Plant Material and growth Conditions.Cariópsides.Plant material Axenic maize seeds (Zea mays L. cv. Apache) were obtained by sterilizing their surface with calcium hypochlorite (7.5 g/100ml MERCK) in the presence of trace amounts of Tween 20 (Aldrich) for 30 minutes, then thoroughly rinsed with distilled water sterilized (120℃, 1 atm, 30 min). After several rinses with axenic distilled water, sterilized seeds were plated into Petri dishes of 8.5 cm in diameter on nutrient-agar semi-solid basal medium (0.7 g Agar/100mL Murashige and Skoog basal solution SIGMA-Hoaglands No.2 at pH 5.7) in and allowed to germinate at 25℃ in developing chamber (Memmert CELSIUS 2000) under 16 h light (110 mmol m-2 s-1) 8 h dark at the same temperature. After 72 h, seedlings were transferred into transplanting hydroponic tubes. The pre-germinated caryopses were transferred aseptically under laminar flow chambers (Burdinola AH-100) to growing cells (Mangenta Vassel GA-7, 350 mL), sterilized at 1 atm, 120℃ for 20 minutes. Glass beads were used as culture medium, (3 mm diameter), representing condition of mechanical impedance. In general, 50 seedlings were used per pot. Plant sterility was checked before and after the experiment by plating out 1 mL aliquots of root-bathing solution on Petri dishes and incubation on nutrient agar. All contaminated plants were immediately discarded.Morphological studyMaize plants were harvested at 4, 6, and 8 days and were carefully removed from their culture medium, thus avoiding fracture of any part of the structure of the root or stem. The following parameters of growth and / or development were recorded:• dry weight biomass of shoot and root. The dry weight was determined by drying to constant weight at 80 ° C in air stream.• Stem length or the second root, which is the length from the base of the epicotyl to the tip of the second root.• Total length of the root. This is the sum of the lengths of the main root, seminal and crowns.Collection root exudatesAt different days of growth, the plants are removed and their roots washed with water. The culture solution and washing the roots are centrifuged, filtered (<0.22 µm), concentrated and re dissolved in 1 mL of methanol with 1% acetic acid for analysis by HPLC.The analysis of exudates in semisolid culture was carried out by removing the culture medium with ethyl acetate (x5). The resulting organic phase is centrifuged, filtered (<0.22 µm) and brought to dryness under reduced pressure. The residue is re dissolved in methanol with 1% acetic acid for analysis of total phenolic content by UV-vis and DIMBOA content by HPLC.Analysis of Hydroxamic Acids in SolutionAll samples were analysed on a Merck Hitachi HPLC equipped with a LaCrom L-7100 quaternary gradient pump, an L-7455 LaChrom diode array detector, and an L-7200 LaChrom autoinjector. Data were collected and processed by using an HPLC data system Merck Hitachi D7000. Instrumental conditions for the analysis of hydroxamic derivatives were as follows: Lichrospher 100 RP-18 (250x4.0 mm, 5 µm) reversed-phase column at 25 ℃; mobile phases were water/1% AcOH (A) and methanol/1% AcOH (B) at a flow rate of 1 mL min-1; injection volume, 50 íL. The following gradient was used for separation: at 0 min, 30% B; 2 min, 30% B; 19 min, 60% B; 21 min, 100% B. Under these conditions the 10.69 min retention times were obtained for DIMBOA, and 16.56 fort MBOA (degradation product of DIMBOA). The detection was carried out at the following wavelengths: 262 and 230 for DIMBOA and MBOA respectively. For quantitative analysis, stock solutions (1 mg/mL) of each individual standard were prepared by dissolving accurate amounts of pure standard in acidified MeOH (1% AcOH). Working standard solutions were obtained by further dilution of stock solutions with MeOH/acidified H2O (1% HOAc) (70:30). These solutions were used to generate the external standard response calibration curves for subsequent measurements of quality parameters and concentration of the hydroxamic acids derivates in solutions at different times. All of the analytical procedures were validated by means of intercalibration laboratory study[55]. Calibration curves for all tested compounds were compared in MeOH (1% v/vacetic acids glacial) and also in Hoagalnd solution (filtered at <0.22 µm.). Optical densities and UV spectra were identical for both conditions (identical molar extinction coefficients), ensuring an identical instrumental response.Analysis of total phenolicTotal phenolic analysis was performed using the colour development reaction between ferric chloride in HCl and OH groups on the aromatic system[56, 57]. The method was developed using as a standard phenolic ferulic acid, a compound common in root exudates of this plant, developing the instruments calibrated by recording the absorbance of the complex formed at different concentrations of ferulic acid at 720 nm. A 1 mL solution of ferulic acid standard or test solution (exudates) was added to 50 mL of distilled water and 3 mL of ammonium ferric sulphate (Aldrich Chemical) were added at 1 min intervals with stirring continuously manually. After exactly 20 min, 3.0 mL of a solution of potassium ferricyanide (Aldrich Chemical), prepared in HCl 0.1 M, were also added in aliquots of 1 mL per minute, with manual stirring. After 20 min, the absorbance was recorded at 720 nm, measured in ultraviolet-visible spectrometer Varian Cary BIO 50, using quartz cells of 2 mL capacity and 1 cm path length. Levels of the corresponding phenol derivatives in the samples of exudates were determined by comparison against a typical graph of concentration - absorbance, generated from the use of ferulic acid as standard.Statistical testsStatistical calculations were performed with the Statistix V 7.0 package, Analytical Software, St. Paul, MN. ANOVA and Tukey tests of comparison were employed.
3. Results
3.1. Morphology of plants
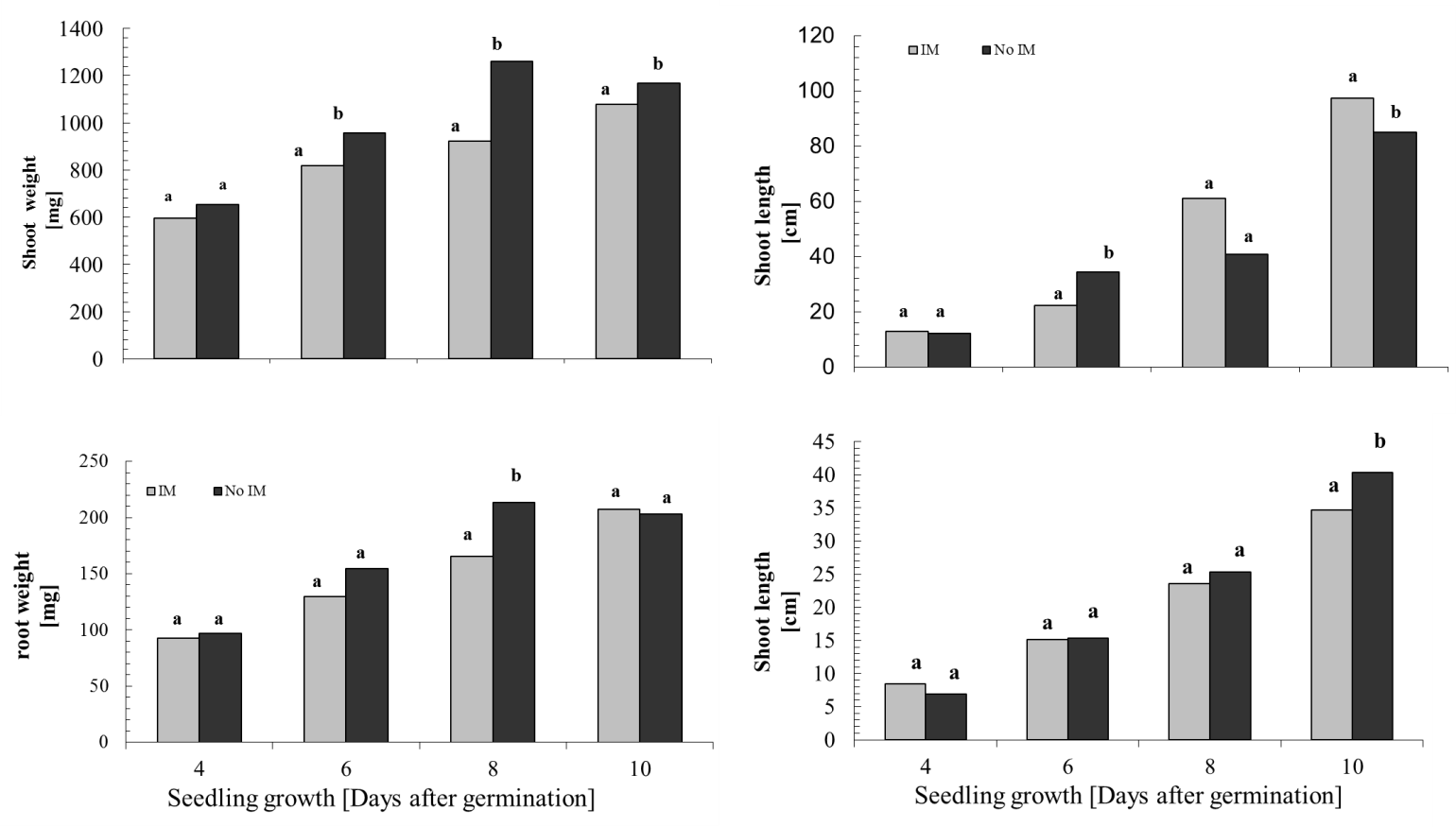
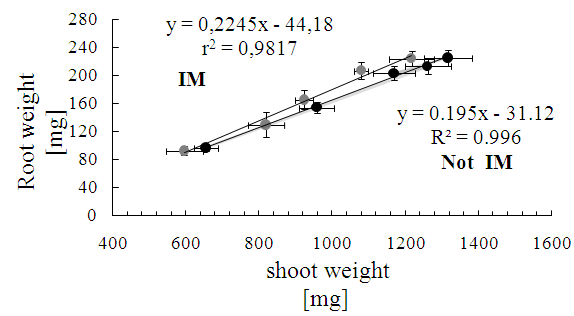
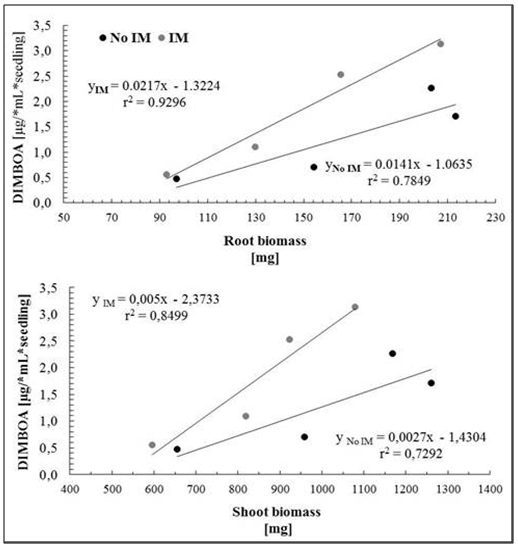
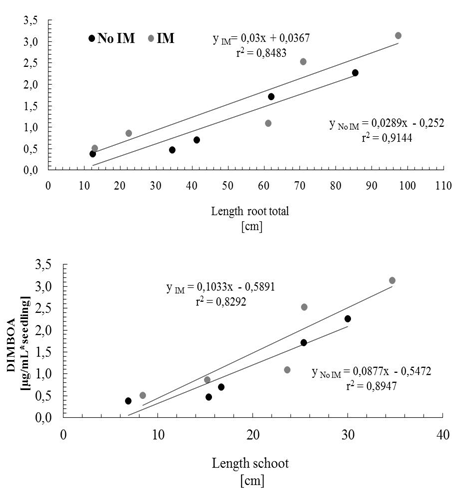
- Controlling the dosage in the use of agar and/or agarose, it is possible to obtain different degrees of IM[57], this system also has the advantage of enabling the same time a direct observation of the root during development, facilitating early detection of changes in anatomical features such as diameter and length, and even perform imaging without further disturbance of the experiment. Despite these advantages, it has questioned the status of the IM by using agar, which has limited its use[58], but recent results show that this culture support, contrasted with the represented with glass beads, cause effects on the morphology of the plant and the total carbon content in exudates associated with an IM effect of different culture media[59]. Consequently, this study has been used in hydroponic culture glass beads as IM and agar solution less IM respect to the glass beads, which were used as control. Given that, the presence of an effect of the IM is recorded by the presence of changes in the morphology of the plant[59], the first phase of this study focused on measuring the characteristic features of plant growth in the two support conditions of cultivation, prevented mechanically (IM) and control (not IM) before quantitative study of the total phenolic compounds DIMBOA and their exudates. The results of the general morphological development of the seedling in half prevented (IM) showed large differences from those with unimpeded growth (not MI), however, there was a delay in issuance and initial leaf expansion in stunted plants (Figure 1).Thus, as shown in Figure 2, there are significant differences in shoot biomass between days 6 and 10. In the case of root biomass is less affected, significant differences were found only on the 8th day of growth after germination. Growth rates of shoot and root also show significant effects according to the growth condition (Figure 3), shoot elongation being slower in condition of IM (p <0.002), finding no difference to the root growth (p> 0.1).The results show that there is an effect of MI on morphological features of the plant, basically expressed in a decrease in biomass accumulation. These effects are actually assigned to the presence in the experimental conditions used MI effect on the growth of the seedling[28, 30]. Close relationships between morphological parameters themselves have been observed.Whatever the level of the impedance, the results thus show correlation between root and shoot characteristics of plant growth. With these results, ensuring the presence of the resistance effect of culture medium used, we proceeded to study the effect these morphological features present in the phenomenon of exudation.
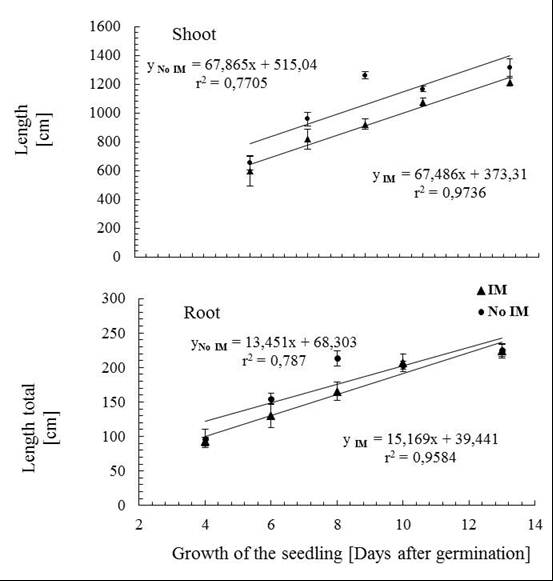 | Figure 3. Relationship between root length and stem growth as time in culture conditions prevented (mechanical impedance-IM) and unimpeded (no mechanical impedance - No IM) |
3.2. Exudates Allelochemical for plant
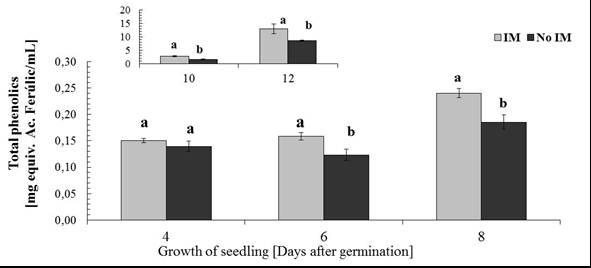
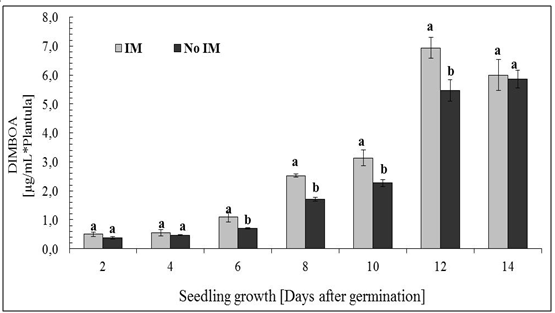
- Phenolic total in root exudateA characteristic feature of cereals is the ability to generate through the root exudates of simple phenolic compounds and hydroxamic acids[60-63], and the latter compound is the most representative DIMBOA in the case of Zea mays L[64]. So these allelochemicals were evaluated in the root exudates according to conditions of IM and not IM. Analyses of total phenolic exudates are shown in figure 4 where differences are observed according to the time of seedling growth. For four days of seedling growth, no significant differences in the amount of total phenolic according to the condition of IM and non IM, after this time, differences are observed until the 12 days of growth. To the extent that the normal plant system is dynamic in terms of its ability to exudation, it is where differences concerning the status of growth can be observed, as are the results obtained for 6 or more days of growth. This phenomenon of increasing concentrations of the components in the exudate is already known, which has been registered changes in the level of total carbon in the exudate, but there is no information on the existence of significant changes in the concentration in family particular compound, as shown here, and even less well documented, changes in production levels of defence compounds (allelochemicals) the effect of IM, as discussed below in the case of DIMBOA, the main defence in Zea mays L. and in gramineae in general.DIMBOA in root exudateAnalysis DIMBOA levels in root exudates were carried out on the same samples of total phenolic analysis. HPLC analyses are shown in Figure 5, where a similar behaviour to that obtained in the analysis of phenolic derivatives. There is a gradual increase in levels of exudates according to the time of seedling growth, not showing significant differences between the 2 and 4 days of growth, but after that time, differences are found for the analysis of 8, 10 and 12 days growth of the seedling. The results again show that an increase in resistance through growth of seedlings results in an increase in the content of DIMBOA in root exudate. In summary, both the phenolic derivatives and DIMBOA, as a constituent of most relevance organic exudates, have important variations in its concentration, increasing with increasing levels of growth medium IM of the roots.
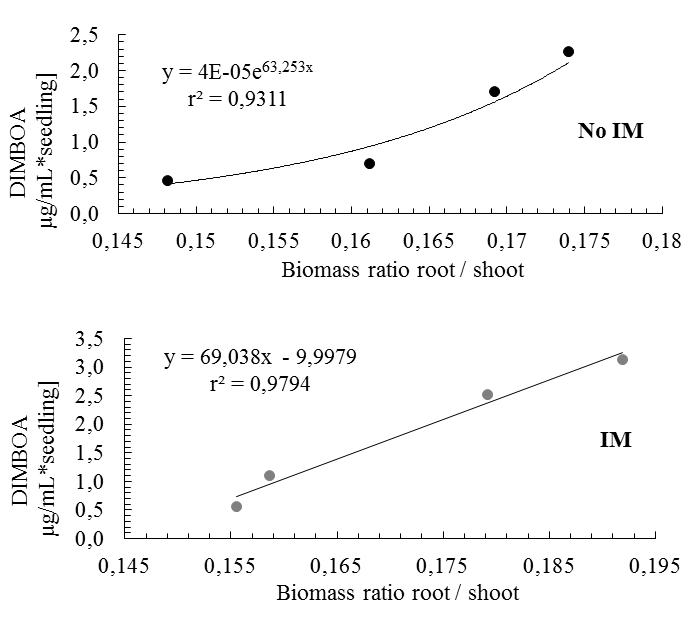 | Figure 8. Relationship between biomass accumulated in root/shoot and DIMBOA in root exudates, as impeded cultivation (mechanical impedance-IM) and unimpeded (no mechanical impedance-No IM) |
4. Conclusions
- In conclusion, the mechanical impedance simulated by glass beads in hydroponic conditions led to significant modifications of whole plant growth, in accordance with changes described by previous works in ‘natural conditions’. Such hydroponic ‘glass bead system’ could therefore be used to test the influence of the impedance on root exudation. Data showed that root exudation was increased by mechanical impedance. Differences in root exudation and morphology between impeded and unimpeded plants really appeared from 6 and 12 days of culture. Results have enabled us to demonstrate the direct influence of impedance on root exudation while the occurrence of an indirect impact of plant morphology on the exudation rate remains probable. Moreover, there are correlations between morphological parameters of the plant and DIMBOA levels in the exudates, which show that all those factors that somehow modify the normal morphological development, affect the levels of compounds in the exudates. This is of relevance in studies evaluating the biological effects of metabolites derived from root exudates, such as allopathic potential. Many experimental designs are carried out in a medium purely hydroponics where the plant is suspended and their roots submerged in the nutrient solution, where IM is zero[10, 47]. In other cases, there is variation in growth conditions, e.g. selecting for seedling assay previously culturing in a culture medium of high IM, such as vermiculite, and after 4 or 5 days of growth, are transplanted by means of bioassay[10, 47] which can be purely hydroponics. Overall, there is growth conditions that may influence the magnitude of the effect measured, where in the case of Zea mays L., conditions of IM generate DIMBOA concentrations between 0.04 M (IM) and 0.03 M (Not IM). These differences may be significant, particularly if the compounds of the exudates showed high biological activity on a host organism. Furthermore, there may be significant changes in the overall balance of C: N in exudates. This may be relevant from the point of view of the microbial population associated with the plant. The importance lies not only in the amount of total carbon, but their relationship off the total nutrient availability-Balance C:N[43], so that an increase in levels of exudation and qualitative variations in composition may affect the microflora associated with the plant, both positively or negatively affects the association between this and the plant and has a major impact on the ecology of the plant.
ACKNOWLEDGEMENTS
- Fellowships from Universidad de Los Andes Venezuela and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Bolsa de Pesquisador Visitante- PV 2011, Brasil (A. O. B.).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML