-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2012; 2(4): 180-185
doi: 10.5923/j.ijaf.20120204.09
Interaction of Imazapyr and a Brassinosteroid Analogue in Seedlings of Eucalyptus grandis
Carlos Magno M. da Silva 1, Silvério de P. Freitas 1, Dra. Mara de M. de A. Gomes 2
1Department of Plant Science, Universidade Estadual do Norte Fluminense Darcy Ribeiro, Campos dos Goytacazes, 28013-600, Brazil
2Department of Plant Physiology, Fundação de Apoio à Escola Técnica do Estado do Rio de Janeiro, Campos dos Goytacazes, 28060-370, Brazil
Correspondence to: Carlos Magno M. da Silva , Department of Plant Science, Universidade Estadual do Norte Fluminense Darcy Ribeiro, Campos dos Goytacazes, 28013-600, Brazil.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
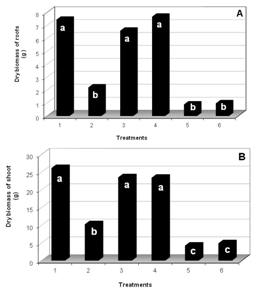
The application of brassinosteroids has been used to protect plants from environmental stresses. An experiment was carried out for the purpose of evaluating the interaction of the spirostanic analogue of castasterone (BB16) and the imazapyr herbicide by making use of seedlings clones of Eucalyptus grandis. It was used manual sprayers for the application of BB16, and for the herbicide application was used a CO2 pressurized backpack sprayer. The experiment was composed of six treatments: control; imazapyr (I) (0.750 kg ha-1 of active ingredient); BB16 in the concentrations of 0.08 (BB16(-)) and 0.16 mg L-1 (BB16(+)) applied immediately after the herbicide (I+BB16(-) and I+BB16(+), respectively) and, imazapyr applied immediately after the BB16 (BB16(-)+I and BB16(+)+I, respectively). At 18 days after the treatment application (DAT) it was verified that in the treatments I, BB16(-)+I and BB16(+)+I occurred total necrosis in terminal leaves of the apical branches. After 21 DAT, the treatment control, I+BB16(-) and I+BB16(+) expressed significative increments in height and stem diameter, in relation to other treatments. Similar behavior was verified to dry matter of the shoot and roots, at 35 DAT. When BB16 was applied before imazapyr, the deleterious effects on the variables dry matter of shoot, as well as in height and diameter of the seedlings, had been more significant than the isolated herbicide application. We found out that the application of analogue castasterona could act as a safener in seedlings of E. grandis submitted to the imazapyr action, when applied immediately after the herbicide, because these seedlings have not exhibited toxicity symptoms under this condition.
Keywords: Brassinosteroid Analogue, Herbicide, Eucalyptus
Article Outline
1. Introduction
- Imazapyr is an herbicide of the imidazolinone family, it is formed by an imidazoline cluster covalently attached to a piridinocarboxilic acid and formulated in isopropylamine salt[1]. This herbicide has broad-spectrum performance[2] used for the control of a broad range of annual and perennial weeds in non-crop vegetation[3], in coniferous forests[4] and in the control of eucalyptus cut stumps[5]. In the latter case, the imazapyr herbicide has high efficiency control (above 90%) and greater flexibility in time between the application and the eucalyptus cutting, in relation to the glyphosate application[6].Imazapyr acts by preventing branched-chain amino acid biosynthesis due to its specific and potent inhibition of acetohydroxyacid synthase (also known as acetolactase synthase). This enzyme participates of the biosynthesis of three aliphatic essential amino acids (present only in plants): valine, leucine and isoleucine[7]; for this reason, theimazapyrherbicide has low toxicity to mammals, with low risks to wildlife[8]. Imazapyr can act in the amino acid pool and in the protein rate; this indicates that the herbicide disrupts protein synthesis by altering the rate of protein turnover[9]. This inhibition interrupts the synthesis of proteins, which, in turn interferes in DNA synthesis and cell growth by causing the death of meristematic regions, it stopsgrowth[10] and interferes in the efficient use of water[11].Some compounds applied to the plant can reduce the damage caused by different types of stress (biotic orabiotic). The brassinosteroids (BRs), for example, can confer tolerance to low and high temperatures, drought, high salt, pathogen attack[12] and photosynthesis inhibitorherbicides[13], mainly due to the induction of enzymaticoxidants[14,15]. Identical tolerance has been observed in relation to the BRs analogues[16,17].Seed soaking treatment with brassinolide (phytosteroid with plant growth-promoting activity) had reduced the phytotoxicity of rice seedling when 2,4-D and butachlor were applied[18].Brassinosteroids are essential to plant development and cause significant responses in growth which includes leaf expansion, activation of proton pumps, stimulationofethylene synthesis (by induction of ACCase), differentiation and cell division, which results in cell elongation[19]. Brassinosteroids are quickly absorbed into the leaf surface, when applied exogenously, and they have a limited transportation. For some authors, these hormones are not transported from original tissue to the others[20], whereas other authors consider this transport only in short distances[21], which involves some hydroxyl groups[22]. On the other hand, imazapyr is quickly absorbed into roots and leaves, and it is translocated from the xylem and phloem to the meristematic tissues[11].Some essays have been conducted in order to show the interrelationship between brassinosteroids and other hormones such as abscisic acid[23], ethylene[24] and jasmonete[25]. However, studies related to the hormonal interaction and products used in commercial plantations are still incipient, especially in relation to herbicides[12]. Therefore, this paper has verified the relationship between BB16 (brassinosteroid analogue) and imazapyr.
2. Materials and Methods
2.1. Requirements
- The E. grandis seedlings were from vegetativepropagation (clone CAF01), three months of age and at a height of 20 to 25 cm; they were cultivated in plastic tubes of 55 cm3, in substrates of carbonized rice shuck and vermiculite, with the proportion of 1:1 v/v. The assay was carried out under greenhouse conditions, in hydroponics system; it was used plastic vases that contained 2,500 ml of nutritive solution, which had the pH corrected every two days to 5.50 ± 0.10, NaOH or HCI was used to the correction. The external side of the vases received silver covering and the vases were involved internally by a transparent plastic coat. The covers of these vases presented perforations of 14 and 2 mm, the first ones were used to receive the eucalypt seedlings and the smaller perforations were destined for the passage of the conducted rubber hose of air to the solution, connected to a compressor with constant airflow.In the transplant, it was used homogenous plants in height which had the root system carefully washed and immediately transferred to the Clark nutritive solution[26]; there was one plant per vase where stayed in a period of 32 days - phase of adaptation to the solution, for emission of new leaves and roots. The nutritive solution was changed weekly, the last change was one day before the foliar application of the compounds. For the BB16 application was used manual spray and for imazapyr application was used carbon dioxide pressurized backpack sprayer regulated to deliver 200 L ha-1 at a constant pressure of 30 lb. pol-2, equipped with hydraulic nozzle of a plane jet Teejet DG95.02 EVS; previously, the vase surfaces were covered with a double-tiered plastic in order to avoid the contamination of the nutritive solution.
2.2. Compounds Used
- The imazapyr herbicide (0.750 kg ha-1 a.i.) was applied before or after the application of 50 mL/seedlings of BB16 (spirostanic analogue of castasterone - (25R)-2α,3α-dihydroxy-5α-espirostan-6-one), in two concentrations (0.08 and 0.16 mg L-1), symbolized by BB16(-) and BB16(+), respectively.
2.3. Data Treatment and Analysis
- The experiment consisted of six treatments: control (no compound application); imazapyr application; BB16, in both concentrations, with the application immediately after the herbicide (I+BB16(-) and I+BB16(+), respectively) and imazapyr, with application immediately after the BB16, this in the concentrations of 0.08 or 0.16 mg L-1 (BB16(-)+I and BB16(+)+I, respectively). When the treatment was constituted of the application of two compounds, the second one was applied only after the leaves had absorbed the first compound.It was used a portable chlorophyll meter (model SPAD- 502 - Soil Plant Analysis Development, Minolta) to the evaluation of the relative chlorophyll content in the third fully expanded leaf (from the apical to the base of the seedling), from the first day of treatment application until 33 days after. The evaluations consisted of five daily measurements (at 8a.m., 10a.m., 12, 2p.m. and 4p.m.); it was taken the average of five measurements of the same leaf in each evaluation time. Weekly evaluations were made on the height (relative to the distance between the collar and the insertion of the youngest leaf) and diameter (measured to 2.5 cm of collar of the seedling) by using a graduated scale and a caliper, respectively, and visual assessments of toxicity that were documented through photography. For dry biomasses determination (35 days after treatment application-DAT) it was carried out the separation of aerial part from roots, so they were placed separately in paper sack, set to the stove with air circulation to 72°± 1℃, until reach constant weight, approximately 72 hours.The statistical design was completely randomized split plot in time (periods of biometric evaluations) with six treatments that corresponds to the control, the dose of the herbicide and its interaction with the doses of BB16 (applied before or after imazapyr), and five replications. Analysis of variance was performed by using the SAS statistical package[27], after verifying the presuppositions and, when the F test was significant, the means were compared by Tukey test to 5% probability.
3. Results
- During the measurement day, the values of the portable chlorophyll meter (SPAD) did not change in relation to the measurement time, similar to that observed in papaya crop[28]. For this reason, the measurements were taken at 4 p.m. as representative of the evaluation day. However, during the experimental period, there was significant variation of SPAD values for the treatments that received the prior application of BB16 (both concentrations), in relation to others.From the eighth DAT it was not possible to take readings of SPAD in the treatment BB16(-)+I due to the total dehydration of the leaf-measure, which was also verified for the treatment BB16(+)+I from 11th DAT (Figure 1). However, in these treatments, there was the beginning of dehydration of the leaves from the tip of the leaf blade, with the symptom evolution toward the leaf base. Due to the SPAD measurement be carried out on the lower third of the leaf blade, it is likely that, with the evolution of the dehydration, the migration of chloroplasts for asymptomatic areas has occurred, such as when the leaves are subjected to stress by light[29], by promoting high concentrations of chlorophyll in these regions, which may have caused increase in SPAD values, checked at the fourth DAT.The SPAD did not find change in leaf-measure colour (third fully expanded leaf) at the treatment that received only the imazapyr application (Figure 1), in spite of changes in other variables. Nevertheless, it was found out that, under the herbicide application, the youngest leaves (apical branches) on the fourth DTA, showed reddish colour on the margin of the leaf blade, which evolved into darkening of the margins, that leads to total blade dehydration of these younger leaves.
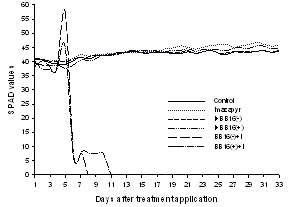 | Figure 1. Values of relative chlorophyll content (SPAD) on the third fully expanded leaf of Eucalyptus grandis seedlings in relation to days after treatment application |
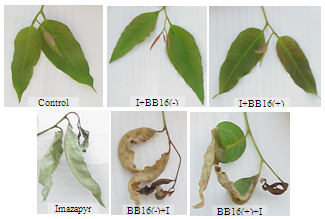 | Figure 2. Visual toxicity symptoms in the leaves of the apical branches of Eucalyptus grandis seedlings at 18 DAT |
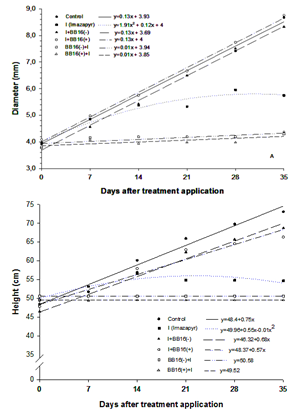 | Figure 3. Diameter (A) and height (B) of Eucalyptus grandis seedlings, over the 35 days of evaluation |
 | Figure 5. Visual toxicity symptoms of imazapyr associated or not to the brassinosteroid analogue application, in Eucalyptus grandis, at 22 DAT |
4. Discussion
- The physiological changes that occur after the imazapyr application include the accumulation of 2-ketobutyrate or derivatives of the inhibition of acetohydroxyacid synthase, amino acid content imbalance, inhibition of DNA synthesis and cell division, and reduction of assimilatetranslocation[30]. However, the effects of AHAS-inhibiting herbicides can be reversed by supplementation with branched chain amino acids (leucine, isoleucine and valine)[31-33].One of the important characteristics of brassinosteroid is its potential to increase the plants tolerance to a wide spectrum of stresses. Despite this, few studies have been conducted to elucidate the mechanism whereby the brassinosteroids promote the tolerance in plants[12]. However, it is known that the brassinosteroids can reduce the phytotoxic effect of herbicides and pesticides by accelerating their metabolism[34]. Thus, BRs can be considered as “safeners” which induce the activity of numerous plant Cyt P450s and enhance glutathione conjugation involved in the biodegradation of herbicides[35].The brassinosteroids can be used as a substrate for enzymes of detoxification, such as sulfotransferases (which is the first step in the process of plant defense against the presence of xenobiotic agents); due to the high affinity between these enzymes and brassinosteroids[36], there may be interaction in the presence of these compounds applied exogenously to the plant. Reference[37] verified that the induction of genes that encodes a sulfotransferase from Brassica napus (BNST) has as preferred substrate the precusors 24-epibrassinolide, 24-epicasthasterone and 24-epiteasterone. The BNST enzymes probably constitute a component of the detoxification of herbicide application in B. napus and the sulfotransferases likely may participate in detoxification reactions of chemicals in some species.It was observed in this assay a complete absence of visual toxicity symptoms in seedlings that received the application of BB16 after imazapyr application, because the BB16 probably have stimulated detoxification mechanisms by involving sulfotransferases, what resulted in a visual aspect similar to control plants.The brassinosteroid receptor (protein BRI1 – brassinosteroid-insensitive-1 - mutant of Arabidopsis brassinosteroid insensitive), which is located in the plasma membrane[38], has an extracellular domain (N-terminal), which is a receptor rich in leucine[39] coupled to a transmembrane domain and a domain serine/threonine kinase (C-terminal) in the cytoplasm, which is activated by phosphorylation and serves to transduction of the signal after the interaction of hormone and the N-terminal domain. Several authors have pointed out that the expression of certain groups of genes is significantly altered by brassinosteroid application, and only the identification of genes that are regulated by this hormone may help to identify the different responses observed in plants treated with brassinosteroid[40,24]. Therefore, the imazapyr application prior to BB16 may have promoted the induction of genes that encode the synthesis of more receptors for the hormone, thus it has increased the binding sites which resulted in greater protective effect on the plant in relation to the chemical stress produced.However, the seedlings that were sprayed with BB16 (with subsequent herbicide application) have shown visual symptoms of toxicity similar to those shown by seedlings under imazapyr application only. Thus, the pretreatment with the analogue castasterona (BB16) has not given tolerance of these plants to imazapyr, although it was applied a few minutes before the herbicide.Similar behaviour has been observed in theinterrelationship between brassinosteroids and pathogenic action of Verticillium dahliae on tomato plants[12]. The inoculated roots with this pathogen which have grown for 14 days in the presence of epihomobrassinolide (EBR), showed no symptoms, or they were of low intensity; however, the plants that were not treated with EBR had moderate or severe symptoms characteristic of infection process of this fungus. The exposure of seedlings for a short time to EBR, before to inoculation of V. dahliae, did not reduce disease symptoms; this is an indicative that EBR was effective only when the pathogen had already been present in plant tissue.The prior application of brassinosteroid may haveinduced the proteins synthesis by stimulating the transcription of mRNA[41] and, as many of these proteins are comprised of branched amino acids leucine, isoleucine and valine, an increase in the quantity of the AHAS content in the cells can have occurred and, consequently, a greater amount of substrate for the action of inhibitor herbicides of this enzyme activity, therefore greater speed of the herbicide action.
5. Conclusions
- In this assay it was found that BB16, a brassinosteroid analogue, had reduced the toxic effects on E. grandis seedlings treated with imazapyr, when the hormone was applied immediately after the herbicide. So, these seedlings have not shown visual symptoms of toxicity and they have not differed statistically from height, diameter and shoot and root system dry biomass to control treatment.The protective action of BB16 on eucalyptus plants treated with imazapyr herbicide can be explained by some hypotheses, but new experimental work will be necessary to clarify these.
ACKNOWLEDGEMENTS
- The authors wish to thank CNPq for the financial support and UENF for the use of the laboratory facilities. We also thank the researcher Miriam Nuñez-Vázquez of Plant Physiology and Biochemistry Department, Instituto Nacional de Ciencias Agrícolas (INCA), who has donated the spirostanic analogue of castasterone to this experiment.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML