-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2012; 2(3): 132-137
doi: 10.5923/j.ijaf.20120203.09
Variation Endogenus and Exogenous of Allelochemical 2,4-dihydroxy-7-metoxy-1,4-benzoxazin-3,(4H)-one (DIMBOA) in Root Architecture of Maize (Zea mayz)
Alberto Oliveros-Bastidas 1, Francisco A. Macías 2, José M.G Molinillo 2
1Laboratorio de Química Ecológica, Departamento de Química, Facultad de Ciencias, Universidad de Los Andes, Núcleo Universitario Pedro Rincón Gutiérrez, La Hechicera. Mérida, 5101, Estado Mérida, Venezuela
2Grupo de Alelopatía, Departamento de Química Orgánica, Facultad de Ciencias, Universidad de Cádiz, C/. República Saharaui s/n, Apdo. 40, 11510, Puerto Real, Cádiz,Spain
Correspondence to: Alberto Oliveros-Bastidas , Laboratorio de Química Ecológica, Departamento de Química, Facultad de Ciencias, Universidad de Los Andes, Núcleo Universitario Pedro Rincón Gutiérrez, La Hechicera. Mérida, 5101, Estado Mérida, Venezuela.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The concentrations of hydroxamic acids 2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3(4H)-one (DIMBOA), in roots and root exudate of 1- to 6- days-old maize plants were determined by high-performance liquid chromatography (HPLC). The highest concentrations of DIMBOA were found in maize root extracts and exudate when maize roots were 2 weeks old and the maize plant was approximately 15 cm in height. The highest concentrations of DIMBOA equivalents were found in root crown. The distribution of DIMBOA in different root parts (seminal roots, primary root, and crown) was also determined. DIMBOA is concentrated in the cortex of all parts of maize roots determined. The concentrations in complete organ of nodal roots were significantly higher than any other parts of maize roots. The results indicate that the distribution of DIMBOA is heterogeneous within architecture of the root. Different groups of root show different potential root exudation of DIMBOA. The level of endogenous DIMBOA directly correlates with exogenous levels. The high concentrations of these substances in the maize root are may be relevant in the resistance of maize varieties to subterranean pest insects.
Keywords: Benzoxazinoids, Defense Molecule, Allelochemicals, Maize (Zea Mays), Root Emergence, Root Exudate, Root-Type
Article Outline
1. Introduction
- Cyclic hydroxamic acids and their derivatives (benzoxazinoids) are major secondary metabolites among poaceous plants. These compounds have biological activity against plants, insects, fungi, and microorganisms (1,2) and could also be involved in detoxification of toxic inorganic molecules (3). The main hydroxamic acid in maize (Zea mays L.) is the 2- ß-D-glucopyranosyloxy-4-hydroxy-7- methoxy-1,4-benzoxazin-3-one (DIMBOA-Glc) (4,5). The benzoxazinoid glucosides are stored in vacuoles as inactive phytoanticipines, while the glucosidases specific for their activation are present in the plastids (6, 7). Upon exogenous or endogenous damage to tissues, the glucoside comes in contact with the glusosidase and the toxic aglucone, DIMBOA (2-4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one), is released (8-10).Benzoxazinoids are considered as constitutive chemical Developing main roots constantly proliferate new lateral roots. Thus, the longitudinal positions of lateral roots along the main root indicate their developmental status, with the younger lateral roots located closer to the root tip. The root tip region contains also many newly initiated lateral root primordial that have been initiated but are not yet visible. Lateral roots and crown roots are formed inside of pre-existing organs and grow out during development. Lateral roots are initiated inside of preexisting roots by resuming mitotic activity of already differentiated cells in the pericycle and sometimes also in the endodermis (25). The lateral roots initially emerge by penetrating the cortex and epidermis of the mother roots. The emerging young lateral roots elongate and mature as o served in other root types. Crown roots initiate inside of the coleoptilar node. Although crown roots originate from the shoot, the fundamental processes of development are similar to those of lateral roots.As well as the shoot, roots are exposed to the ambience of the soil, and can be subjected to different types of stress such as fungi, nematodes, bacteria and parasitic plants. Thus, in the root architecture mechanisms of response are different, mainly the production of antimicrobial metabolites, inactivation of proteins derived from pathogens, among other mechanisms. Moreover, it is known that some allelochemicals differentially affect the development the different groups in maize root. For example, coumarin, a allelochemicals widely distributed in the plant kingdom, inhibited root length, but effects differed depending on the root type en maize (26).In this communication, we report the variation of levels endogenous and exogenous release of benzoxazinoids and discuss possible explanations for the relationship between defense substances and the evolution of root emergence. The information obtained ought to advance our understanding of how plants adapt to soil environmental stresses, such as allelochemicals distribution in roots. Thus, for a better understanding of the effects of allelochemicals on plant roots, the morphological variability within a root system in its potential to release allelochemicals needs to be studied.. You will not need to remember shortcut keys. Just a mouse-click at one of the menu options will give you the style that you want.
2. Materials and Methods
- Plant Material and growth Conditions.CariópsidesPlant material Axenic maize seeds (Zea mays L. cv. Apache) were obtained by surface sterilizing for sterilized in their surface by calcium hypochlorite (7.5 g/100ml MERCK) in the presence of trace amounts of Tween 20 (Aldrich) for 30 minutes, then thoroughly rinsed with distilled water sterilized (120 °C, 1 atm, 30 min). After several rinses with axenic distilled water, sterilized seeds were plated Petri dishes 8.5 cm in diameter on nutrient-agar semi-solid basal medium (0.7 g Agar/100mL Murashige and Skoog basal solution SIGMA-Hoaglands No.2 at pH 5.7) in and allowed to germinate at 25 °C in developing chamber (Memmert CELSIUS 2000) under 16 h light (110 mmol m-2 s-1) 8 h dark at the same temperature. After 72 h, seedlings were transferred into transplanting hydroponic tubes. The pre-germinated caryopses were transferred aseptically under laminar flow chambers (Burdinola AH-100) to growing cells (Mangenta Vassel GA-7, 350 mL), sterilized at 1 atm, 120 ° C for 20 minutes. Were used as culture medium, beads glass (3 mm diameter). In general were used 50 seedlings per pot. Plant sterility was checked before and after the experiment by plating out 1 mL aliquots of root-bathing solution on Petri dishes and incubation on nutrient agar. All contaminated plants were immediately discarded. Exudates according to the type of rootTo capture and analysis of exudates from different root types of maize, during the growth and emergence of the roots, they are oriented manually to different reservoirs with nutritive solution, so that their development is carried out within the compartments created, and at end of test, the solutions of each of these reservoirs are analysed in content of DIMBOA.Morphological study NomenclatureAccording to Hochholdinger, et al. 2004, where root system of maize can be divided into an embryonic root system consisting of a single primary root or radicle and a variable number of seminal roots, and a post-embryonic root system which is made up by shoot-borne roots (Figure 1). Shoot-borne roots formed at consecutive underground nodes are called crown roots. Lateral roots which emerge from all major root-types also belong to the post-embryonic root system.Treatment seedling rootMaize plants were harvested between 8 and 10 days were carefully removed from their culture medium, thus avoiding fracture of any part of the structure of the root or stem. The following parameters of growth and / or development were recorded:• dry weight biomass of shoot and type root. The dry weight was determined by drying to constant weight at 80 ° C in air tream.• Shoot length primary, seminal and crowns roots.• Total length of the root. This is the sum of the lengths of the main root, seminal and crowns.Collection root exudatesAt different days of growth, the plants are removed and their roots washed with water. The culture solution and washing the roots are centrifuged, filtered (<0.22 µm), concentrated and re dissolved in 1 mL of methanol with 1% acetic acid for analysis by HPLC.Analysis of Hydroxamic Acids in SolutionAll samples were analysed on a Merck Hitachi HPLC equipped with a LaCrom L-7100 quaternary gradient pump, an L-7455 LaChrom diode array detector, and an L-7200 LaChrom auto injector. Data were collected and processed by using an HPLC data system Merck Hitachi D7000. Instrumental conditions for the analysis of hydroxamic derivatives were as follows: Lichrospher 100 RP-18 (250x4.0 mm, 5 µm) reversed-phase column at 25 °C; mobile phases were water/1% AcOH (A) and methanol/1% AcOH (B) at a flow rate of 1 mL min-1; injection volume, 50 µL. The following gradient was used for separation: at 0 min, 30% B; 2 min, 30% B; 19 min, 60% B; 21 min, 100% B. Under these conditions the 10.69 min retention times were obtained for DIMBOA, and 16.56 fort MBOA (degradation product of DIMBOA). The detection was carried out at the following wavelengths: 262 and 230 for DIMBOA and MBOA respectively. For quantitative analysis, stock solutions (1 mg/mL) of each individual standard were prepared by dissolving accurate amounts of pure standard in acidified MeOH (1% AcOH). Working standard solutions were obtained by further dilution of stock solutions with MeOH/acidified H2O (1% HOAc; 70:30). These solutions were used to generate the external standard response calibration curves for subsequent measurements of quality parameters and concentration of the hydroxamic acids derivates in solutions at different times. All of the analytical procedures were validated by means of intercalibration laboratory study (27).
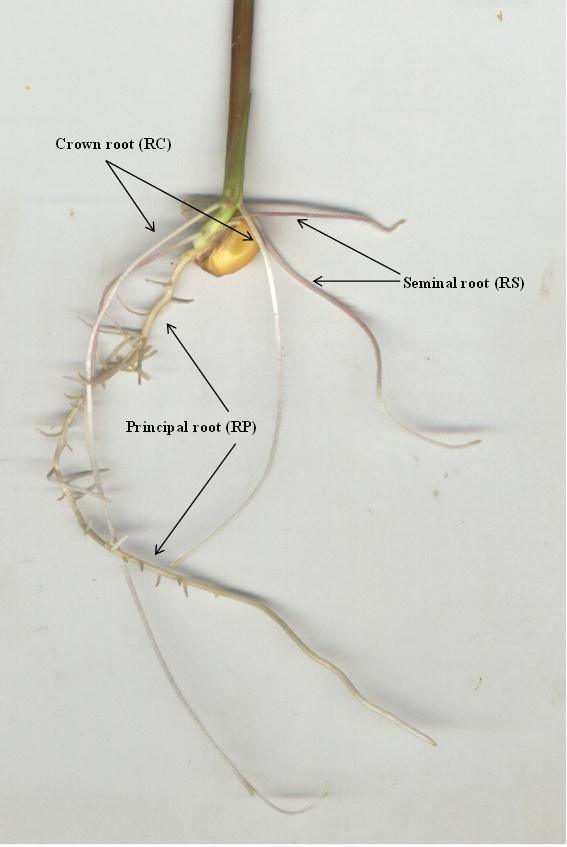 | Figure 1. Structure and nomenclature of the maize root system. |
3. Result and Discussion
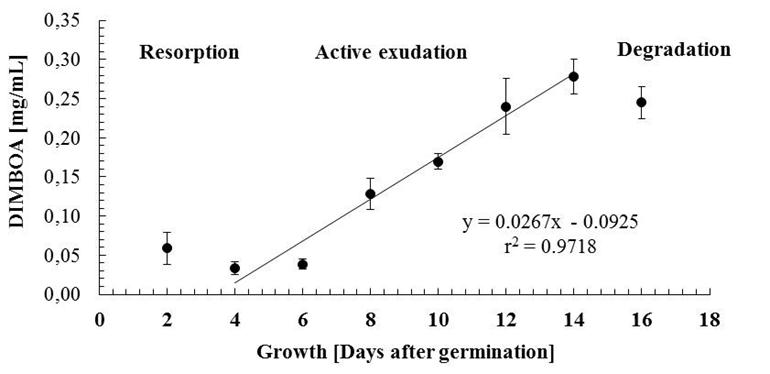
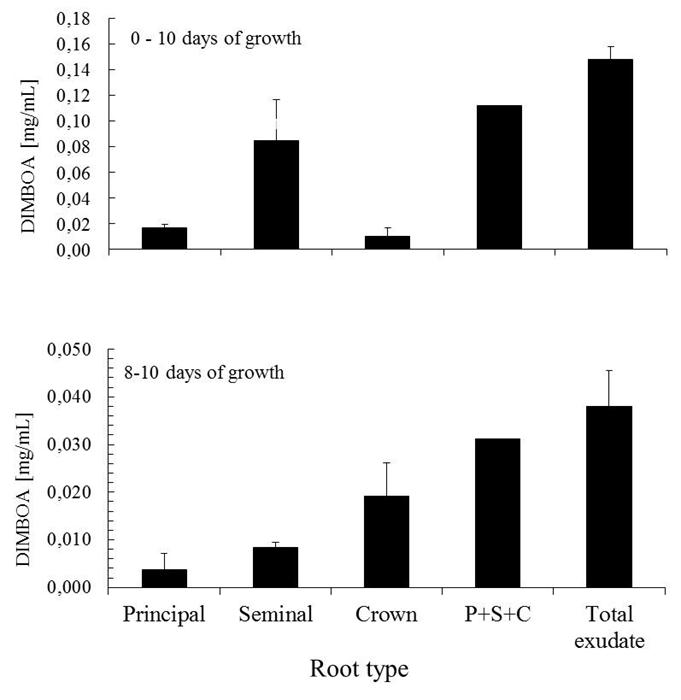
- DIMBOA total in root exudate during growthHas been observed that for some species, the distribution of metabolites in the root is not uniform, accumulating preferentially in those sections emerging and rapidly growing, where the root morphological factors can be responsible for this distribution. In this regard, a histochemical study conducted on the presence of benzoxazinoids on the surface of lateral roots and crowns of wheat show that the accumulation of these compounds is differential within the architecture of the root, and has been proposed that this accumulation is a strategy of defense against pathogen infection, especially in emergent tissue (10). With this background we evaluated the accumulation of DIMBOA, both endogenous and exogenous, in the different roots of maize seedlings.The Fig-2 shows the accumulation of DIMBOA in the root exudates during the time of seedling growth. Two characteristics of the curve are observed.
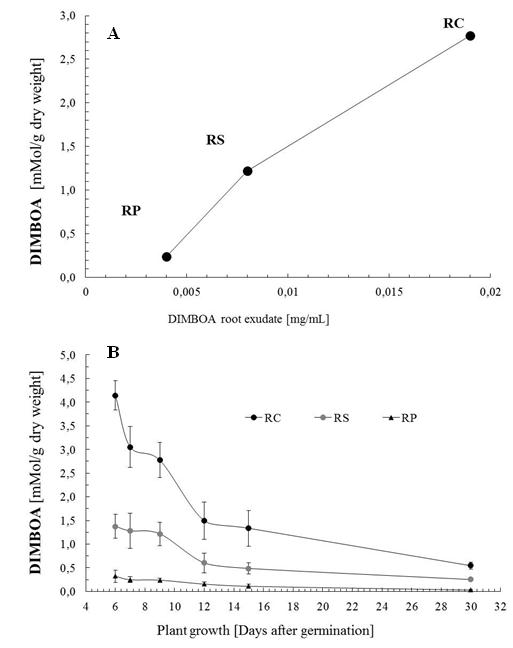
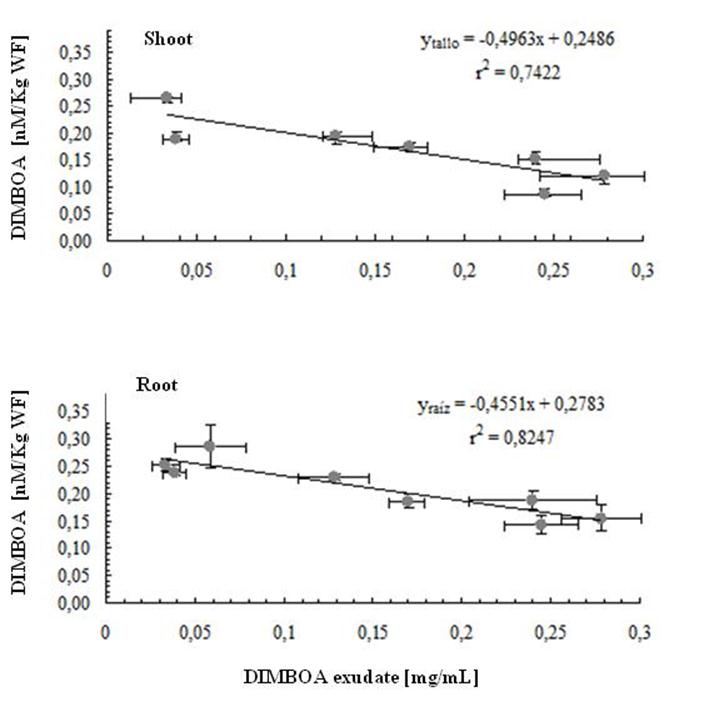 | Figure 3. Relationship between DIMBOA levels of endogenous (Roor and Shoot) and exogenous (root exudates) for the growth of Zea mays. |
5. Conclusions
- Many studies of the maize plant were carried out with mutant species, plants with a defect (mutation) in at least one of their genes. Many of these mutants have been recognized for its ability to develop with particular morphological features at its root, such as maize mutant rtcs-1 and rtcs-2 (rootless from crown and seminal roots), are characterized by absence of crown and seminal roots and the plant depends solely on its primary root, and can still grow to their mature state and be fertile. For these mutants, which have been subjected to studies of their development from the point of view morphological and agricultural sustainability is interesting to consider the consequences of the loss of chemistry information by the absence of their root systems. Their resistance to other plant species (bad herbs) and infection (aphids, fungi and other microorganisms) can be affected. For example, many of these mutants show variations in symbiosis with mycorrhizal induction, affecting significantly the development of the plant, and potentially its manufacturing productivity. This may be due to the absence of antibiotic compounds of the type alkaloid, here studied. The accumulation of such allelochemicals seems to be a very effective way of avoiding pathogenic infection during root emergence. Considering that allelochemicals are spatially concentrated in top soil layers where the sensitive seminal roots generally grow, DIMBOA could have an important ecological impact, influence the composition of organisms within a plant community.
ACKNOWLEDGEMENTS
- Fellowships from Universidad de Los Andes (ULA) Venezuela and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Bolsa de Pesquisador Visitante- PV 2011, Brasil (A. O. B.).
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML