| [1] | Sarhan, A.R.T., Sharif, F.M., 1986. Integrated control of Fusarium wilt of pepper. Acta phytopathologica of Entomologica Hungarica, 21(1-2), 123-126. (c.f. R. Pl. Pathol., 67,2191). |
| [2] | lbrahimllari, L., 1987. Some data on wilt organisms of pepper in the district of Tirane. Buletini i shkencave Bujqesore, 26 (3), 94-100. (c.f. R. Pl. Pathol, 67, 3229). |
| [3] | Koleva, K., Vitanov, M., 1990, Fusarium species related to root rot of pepper. Rasteniev dni Nauki 26 (6), 61-63. (c.f. R. Pl. Pathol, 72 (3), 1559). |
| [4] | Camino, V., Depestre, T., Espinosa, J., 1990, Search for Capsicum susceptibility to Fusarium. Capsicum Newsletter, 6, 70. (c.f. R. Pl. Pathol., 70 (4), 2406). |
| [5] | Jones, M.M., Black, L.L., 1992. Sources of resistance among Capsicum spp. to Fusarium wilt of pepper. Capsicum Newsletter, (11), 33-34. (c.f. R. Pl. pathol., 72 (9) 6121). |
| [6] | Abdel-Kader, M.M., 1999, Biological and chemical control of wilt disease of hot pepper capsicum annum L. Egypt. J. Phtopathol., 27 (1), 1-8. |
| [7] | Abdel-Monaim, M. F., Ismail, M.E., 2010, The Use of Antioxidants to Control Root Rot and Wilt Diseases of Pepper. Not. Sci. Biol., 2 (2), 46-55. |
| [8] | Fegla, G.I.A., 1966, Seed treatment and soil drench with certain fungicides as a control measure for damping-off diseases of some solanaceous plants. M.Sc., thesis, Fac. Agric., Alex. Univ., 100pp. |
| [9] | Liang, L.Z., 1990, Seed-borne Fusarium of Chilli and their pathogenic significance. Actaphytopathologica sinica, 20(2), 117-121. (c.f. R. Pl. Pathol, 73 : 4490). |
| [10] | Sivan, A., 1987, Biological control of Fusarium crown rot of tomato by Trichoderma harzianum under field conditions. Plant Dis., 71, 587–592. Doi: 10.1094/PD-71-0587 |
| [11] | Punja, Z.K., Utkhede, R.S., 2004, Biological control of fungal diseases on vegetable crops with fungi and yeasts. In: Fungal Biotechnology in Agricultural, Food, and Environmental Applications (ed. D.K. Arora), New York Basel, pp. 157-171. |
| [12] | Punja, Z. K., Grogan, R.G., 1982, Effects of inorganic salts, carbonate-bicarbonate anions, ammonia, and the modifying influence of pH on sclerotial germination of Sclerotium rolfsii. Phytopathology, 72, 635-639. |
| [13] | Smilanick, J.L., Margosan, D.A., Mlikota, F., Usall, J., Michael, I.F., 1999, Control of Citrus Green Mold by Carbonate and Bicarbonate Salts and the Influence of Commercial Postharvest Practices on Their Efficacy. Plant Disease, 83, 139-145. |
| [14] | El-Gamal, N.G., Abd-El-Kareem, F., Fatooh, Y.O., El-Mougy, N.S., 2006, Induction of Systemic Resistance in Potato Plants Against Late and Early Blight Diseases Using Chemical Inducers under Greenhouse and Field Conditions. Research Journal of Agriculture and Biological Sciences, 3(2), 73-81. |
| [15] | Abd-El-Kareem, F., 2007, Potassium or sodium bicarbonates in combination with Nerol for controlling early blight disease of potato plants under laboratory, greenhouse and field conditions. Egypt. J. Phytopathol., 35, 73- 86. |
| [16] | Ragab, M.M.M., Saber, M.M., El-Morsy, S.A., Abd El-Aziz, A.R.M., 2009, Induction of Systemic Resistance Against Root Rot of Basil Using Some Chemical Inducers. Egypt. J. Phytopathol., 37 (1), 59-70. |
| [17] | Sivropoulou, A., Kokkini, S., Lanaras, T., 1995, Antimicrobial activity of mint essential oil. Journal of Agricultural and Food chemistry, 43, 2384-2388. |
| [18] | Sivropoulou, A., Nicolaou, C., Papanikolaou, E., Dokkini, S., Lanaras, T., Arsenakis, M., 1997, Antimicrobial, cytotoxic and antiviral activities of Saliva fruiticosa essential oil. Journal of Agricultural and Food chemistry, 45, 3197-3201. |
| [19] | Hammer, K.A., Carson, C.F., Riley, T.V., 1999, Antimicrobial activity of essential oils and other plant extracts. J. Appl. Microbiol., 86, 985-990. |
| [20] | El-Mougy, N.S.; Abd-El-kareem, F.A.; El-Gamal, N.G. and Fotouh, Y.O. 2004. Application of fungicides alternatives for controlling cowpea root rot disease under greenhouse and field conditions. Egypt. J. Phytopathol., 32 (1-2), 23-35. |
| [21] | Ferreira, J.H.S., Matthee, F.N., Thomas, A.C., 1991, Biological control of Eutypa lata on Grapevine by an antagonistic strain of Bacillus subtilis. Phytopathology, 81, 283-287. |
| [22] | SAS 1996. Statistical Analysis System. User‛s Guide: Statistics (PC-Dos 6.04). SAS Institute Inc., Cary, NC, USA. |
| [23] | Winer B.J. 1971. Statistical Principles in Experimental Design. 2nd ed. MiGraw-Hil Kogakusha, LTD, 596 pp. |
| [24] | Bell, D.K., Wells, H.D., Markham, B.B., 1982, In vitro antagonism of Trichoderma species against six fungal plant pathogens. Phytopathology, 72, 379--382. |
| [25] | Abdel-Kader, M.M., 1997, Field application of Trichoderma harzianum as biocide for control bean root rot disease. Egypt. J. Phytopathol., 25, 19--25. |
| [26] | El-Mougy, N.S., 2001, Field application of certain biological and chemical approaches on controlling Bean wilt disease. Egypt. J. Phytopathol., 29, 69--78. |
| [27] | Papavizas, G.C., 1985, Trichoderma and Gliocladium: biology, ecology, and potential for biocontrol, Annu. Rev. Plant Pathol., 23, 23-54 |
| [28] | Hjeljord, L., Tronsmo, A., 1998, Trichoderma and Gliocladium: enzymes, biological control and commercial applications, vol. 2. London: Taylor and Francis, Ltd. Trichoderma and Gliocladium in biological control: an overview. p. 131–151. |
| [29] | Chet, I., Benhamou, N., Haran, S., 1998, Trichoderma and Gliocladium: enzymes, biological control and commercial applications, vol. 2. London: Taylor and Francis, Ltd. Mycoparasitism and lytic enzymes. p. 153–172. |
| [30] | Singh, P.P., Shin, Y.C., Park, C.S., Chung, Y.R., 1999, Biological control of Fusarium wilt of cucumber by chitinolytic bacteria. Phytopathology. 89:92–99. |
| [31] | Weller, D.M., 1988, Biological control of soilborne plant pathogens in the rhizosphere with bacteria. Annu. Rev. Phytopathol., 26,379-407. |
| [32] | Leeman, M., Van Pelt, J.A., Den Ouden, F.M., Heinsbroek, M., Bakker, P.A.H.M., Schippers, B., 1995, Induction of systemic resistance against Fusarium wilt of radish by lipopolysaccharides of Pseudomonas fluorescens. Phytopathology, 85, 1021-1027. |
| [33] | Alabouvette, C., Lemanceau, P., Steinberg, C., 1996, Use of nonpathogenic Fusarium oxysporum and fluorescent Pseudomonas to control Fusarium wilts. Pages 155-164 in: Proc. Int. Workshop Biol. Control Plant Dis. T. Wenhua, R. J. Cook, and A. Rovira, eds. Hokkaido University, Sapporo, Japan. |
| [34] | Kim, D.S., Cook, R.J., Weller, D.M., 1997, Bacillus sp. L324--92 for biological control of three root diseases of wheat grown with reduced tillage. Phytopathology, 87, 551--558. |
| [35] | Parke, J.L., Rand, R.E., Joy, A.E., King, E.B., 1991, Biological control of Pythium damping-off and Aphanomyces root rot of peas by application of Pseudomonas cepacia or P. fluorescens to seed. Plant Disease, 75, 987-992. |
| [36] | Al-Zaemey, A.B., Magan, N., Thompson, A.K., 1993, Studies on the fruit coating polymers and organic acid on growth of Colletotrichum musae in vitro and postharvest control of anthracnose of bananas. Mycological Res., 97, 1463 – 2468. |
| [37] | Olivier, C., Macneil, C.R., Loria, J., 1999, Application of organic and inorganic salts to field-grown potato tubers can suppress silver scurf during potato storage. Plant Dis., 83, 814 – 818. |
| [38] | Ryu, D., Hold, D.L., 1993, Growth inhibition of Penicilium expansum by several commonly used food ingredients. J Food Protection, 56, 862 – 867. |
| [39] | Saleh, O.I., Huang, J.S., 1997, Bacterial soft rot disease of tomato fruits in Florida, USA: Identification, response of some American and Egyptian cultivars of solanaceous plants and chemical control. Assuit J. Agric. Sci ., 28, 11 – 26. |
| [40] | Olivier, C., Halseth, D.E., Mizubuti, E.S.G., Loria, J., 1998, Postharvest application of organic and inorganic salts for suppression of silver scurf on potato tubers. Plant Dis., 82, 213 – 217. |
| [41] | Hall, D.J., 1992, Comparative activity of selected food preservatives as citrus postharvest fungicides. Horticultural Society, 101,184 – 187. |
| [42] | Fente, C.A., Vazquez, B.B., Franco, A.C., Quinto, F.E., 1995, Distribution of fungal genera in cheese and dairies: Sensitivity to potassium sorbate and natamycin. Arichiv fur Lebensmitt Elhygiene, 46, 62 – 65. |
| [43] | Marin, S., Guynot, M.E., Sanchis, V., Arbones, J., Ramos, A.J., 2002, Aspergillus flavus, Aspergillus niger, Penicillium corylophillum spoilage prevention of bakery products by means of weak acid preservatives. J. Food Sci., 67, 2271–2277. |
| [44] | Stromberge, A., Brishammar, S., 1991, Induction of systemic resistance in potato (Solanum tuberosum L.) plants to late blight by local treatment with Phytophthora infestans, Phytophthora cryptogea or di-potassium phosphate. Potato Res., 34, 219-225. |
| [45] | Yurina, T.P., Karavaev, V.A., Solntsev, M.K., 1993, Characteristics of metabolism in two cucumber cultivars with different resistance to powdery mildew. Russian Plant Physiol., 40, 197-202. |
| [46] | Mucharromah, E., Kuc, J., 1991, Oxalate and phosphates induced systemic resistance against diseases caused by fungi, bacteria and viruses in cucumber. Crop Protection, 10, 265-270. |
| [47] | Reuveni, M., Agapov V., Reuveni, R., 1995, Suppression of cucumber powdery mildew (Sphaerotheca fuliginea) by foliar sprays of phosphate and potassium salts. Plant Pathol., 44, 31-39. |
| [48] | Ibrahim, I.A., El-Zarka, A.M., Assal, W.N., Abou El-Ela, W.M., 1976, Control of root-rot disease of Roselle by certain fungicides. Proc. 2nd Conf Phytopathol, Cairo, pp 865-880. |
| [49] | Ziedan, S.H.S., 1993, Studies on Fusarium wilt disease of sesame in A.R.E. M. Sc. Thesis, Fac. Agric., Ain shains Univ., 176pp. |
| [50] | El-Mougy, N.S., 1995, Studies on wilt and root rot diseases of tomato in Egypt and their control by modem methods. M.Sc. thesis, Fac. Agric., Cairo Univ., 127pp. |
| [51] | Smilanick, J.L., Mansour, M.F., Sorenson, D., 2006, Pre- and postharvest treatments to control green mould of citrus fruit during ethylene degreasing. Plant Dis., 90, 89-96. |
| [52] | Kuchitsu, K., Kikuyama, M. Shibuya, N., 1993, N-Acetylchito-oligosaccharides, biotic elicitor for phytoalexin production, induce transient membrane depolarization in suspension-cultured rice cells. Protoplasma, 174, 79-81. |
| [53] | Bell, A.A., Hubbard, J.C., Liu, L., Davis, R.M., Subbarao, K.V., 1998, Effects of chitin and chitosan on the incidence and severity of Fusarium yellows of celery. Pl. Dis., 82, 322-328. |
| [54] | Abd-El-Kareem, F., 2002, Integrated treatments between bioagents and chitosan on root rot diseases of pea plants under field conditions. Egypt J. Appl. Sci., 17, 257-279. |
| [55] | Leuba, J.L., Stossel, P., 1986, Chitosan and other polyamines: Antifungal activity and interaction with biological membranes. In Muzzarelli, R. and Goody, G.W.(eds.), Chitin in nature and technology. Plenum Press, New York, pp: 215-222. |
| [56] | Abd-El-Karem, F., El-Mougy, N.S., El-Gamal, N.G., Fotouh, Y.O., 2006, Use of chitin and chitosan against tomato root rot disease under greenhouse conditions. Research Journal of Agriculture and Biological Sciences, 2(4), 147-152. |
| [57] | Hirano, S., Itakura, C., Seino, H., Akiyama, Notata, I., Kanbara, N., Kawakami, N., 1990, Chitosan as an ingredient for domestic animal feeds. J. Agric. Food Chem., 38, 1214-1217. |
| [58] | Leuba, J.L., Stossel, P., 1986, Chitosan and other polyamines: Antifungal activity and interaction with biological membranes. In Muzzarelli, R. and Goody, G.W.(eds.), Chitin in nature and technology. Plenum Press, New York, pp: 215-222. |
| [59] | Hadwiger, L.A., Loschke, D.C., 1981, Molecular communication in host-parasite interactions: Hexosamine polymers) chitosan (as regulator compounds in race-specific and other interactions. Phytopathology, 71, 756-762. |
| [60] | Kaur J., Arora D., 1999, Antimicrobial activities of species. Int. J. Antimicrob. Agents, 12, 257–262. |
| [61] | Naqui, S.H.A., Khan M.S.Y., Vohora S.B., 1994, Antibacterial, antifungal and anthelmintic investigation on Indian medicinal plants. Fitoterapia, 62, 221–228. |
| [62] | Nascimento, G.G., Locatelli, J., Freitas, P.C., 2000, Antibacterial activity of plants extracts and phytochemical on antibiotic resistant bacteria. Braz. J. Microbiol., 31, 247–256. |
| [63] | Nychas, G.J.E., 1996, Natural antimicrobial from plants. p. 235–258. In: “New Methods of Food Preservation” (G.W Gould, ed.). Londres, CRC Press. |
| [64] | Karapinar, M., 1985, The effects of citrus oil and some Turkish spices on growth and aflatoxin production by Aspergillus parasiticus NRRL 2999. Int. J. Food Microbiol., 12, 239–245. |
| [65] | Nanir, S.P., Kadu B.B., 1987, Effect of some medicinal plants extract on some fungi. Acta Botanica India, 15, 170–175. |
| [66] | Nirmala, K., Singh, S.K., Dubey, N.K., 1988. Fungitoxic activity of essential oil of Juniperus communis. Indian Perfunm, 33, 25–29. |
| [67] | Juglal, S., Govinden, R., Odhav, B., 2002, Spices oils for the control of co-occurring mycotoxin-producing fungi. J. Food Protect., 65, 638–687. |
| [68] | Campo, J., Nguyen-The, C., Sergent, M., Amito, M.J., 2003, Determination of most bioactive phenolic compounds from rosemary against Listeria monocytogenes: influence of concentration, pH and NaCl. J. Food Sci., 68, 2066–2071. |
| [69] | Brull, S., Coote, P., 1999, Preservative agents in foods: mode of action and microbial resistance mechanisms. Inter. J. Food Microbiol. 50, 1–17. |
| [70] | Juven, B.J., Kanner, J., Sched, F., Weisslowicz, H., 1994, Factors that interact with the antibacterial of thyme essential oil and its active constituents. J. Appl. Microbiol., 76, 626–631. |
| [71] | Kim, J., Marshall, M.R., Wei, C., 1995, Antibacterial activity of some essential oils components against five food-borne pathogens. J. Agric. Food Chem., 43, 2839–2845. |

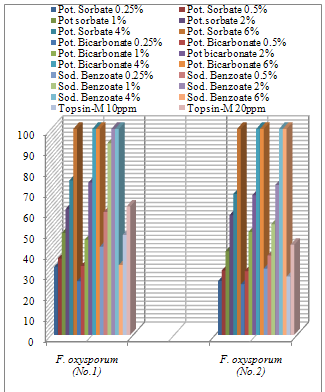
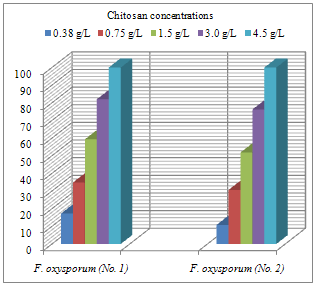
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML