-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
International Journal of Agriculture and Forestry
p-ISSN: 2165-882X e-ISSN: 2165-8846
2012; 2(1): 115-127
doi:10.5923/j.ijaf.20120201.18
Different Approaches of Bio-control Agents for Controlling Root Rot Incidence of Some Vegetables under Greenhouse Conditions
M. M. Abdel-Kader, Nehal S. El-Mougy, M. DE. Aly Lashin
Plant Pathology Department, National Research Centre, Giza, Egypt
Correspondence to: M. M. Abdel-Kader, Plant Pathology Department, National Research Centre, Giza, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Different approaches of some antagonistic fungal, bacterial and yeast agents applied as seed treatment or soil drench was evaluated against various soil-borne pathogens causing vegetables root rot disease under greenhouse conditions. The tested pathogenic fungi were Alternaria solani Fusarium solani, F. oxysporum, Rhizoctonia solani, Sclerotium rolfsii, Macrophomina phaseolina and Pythium sp., meanwhile the tested bio-agents were Trichoderma harzianum, T. Viride andBacillus subtilis, Pseudomonas flourescens and Sacchromyces serivisae. Significant reduction in the disease incidence was observed in bio-agent treatments in comparison with untreated control. Root rot incidence, at pre-emergence stage, significant effect was observed in bio-agent treatments as seed soaking comparing with soil drench treatment. The treated seeds showed a protective effect for seeds germination against the invasion by soil-borne pathogenic fungi.Meanwhile,soil drenched with different bio-agents showed more efficacy for reducing root rot incidence at post-emergence growth stage of tested vegetables, Cucumber, Cantaloupe, Tomato and Pepper. The obtained results revealed that the antagonist T. harzianum showed significant superior effect to reduce diseases incidence followed by B. subtilis. Also, the antagonists T. viride and P. fluorescence occupied significantly the second degree for reducing root rot incidence. The treatment with S. serevisiae had the lowest effect on disease incidence, although it significantly lesser than check control treatment.The present study demonstrates that application of bio-agentsas seed treatment and soil drench may be useful for controlling root rot disease in field.
Keywords: Root Rot, Seed Dressing, Soil Drench, Bio-Agents, Cucumber, Cantaloupe, Tomato, Pepper
Cite this paper: M. M. Abdel-Kader, Nehal S. El-Mougy, M. DE. Aly Lashin, Different Approaches of Bio-control Agents for Controlling Root Rot Incidence of Some Vegetables under Greenhouse Conditions, International Journal of Agriculture and Forestry, Vol. 2 No. 1, 2012, pp. 115-127. doi: 10.5923/j.ijaf.20120201.18.
Article Outline
1. Introduction
- Vegetable crops are grown worldwide as a source of nutrients and fiber in the human diet. Fungal plant pathogens can cause devastation in these crops under appropriate environmental conditions. Vegetable producers confronted with the challenges of managing fungal pathogens have the opportunity to use fungi, bacteria and yeasts as biological control agents. Several commercially available products have shown significant disease reduction through various mechanisms to reduce pathogen development and disease. Plant diseases need to be controlled to maintain the quality and abundance of food, feed, and fiber produced by growers around the world. Different approaches may be used to prevent, mitigate or control plant diseases. Beyond good agronomic and horticultural practices, growers often rely heavily on chemical fertilizers and pesticides. Such inputs to agriculture have contributed significantly to the spectacular provements in crop productivity and quality over the past 100 years. However, the environmental pollution caused by excessive use and misuse of agrochemicals, as well as fear-mongering by some opponents of pesticides, has led to considerable changes in people’s attitudes towards the use of pesticides in agriculture.Today, there are strict regulations on chemical pesticide use, and there is political pressure to remove the most hazardous chemicals from the market. Additionally, the spread of plant diseases in natural ecosystems may preclude successful application of chemicals, because of the scale to which such applications might have to be applied. Consequently, some pest management researchers have focused their efforts on developing alternative inputs to synthetic chemicals for controlling pests and diseases. Among these alternatives are those referred to as biological control. The application of biological controls using antagonistic microorganisms has proved to be successful for controlling various plant diseases in many countries[1]. However, this is not an easy method, and it is costly to apply; however it can serve as the best control measure under greenhouse conditions. Trichoderma harzianum introduced to the soil, was able to reduce root rot incidence of faba bean plants significantly more than the fungicide Rizolex-T[2]. In recent years, several attempts have been made to overcome this obstacle by applying antagonistic microorganisms. Trichoderma spp. are well documented as effective biological control agents of plant diseases caused by soilborne fungi[3-5]. Many investigators[6-8] observed that the application of wheat bran colonised by T. harzianum to soil infested with R. solani and S. rolfsii, reduced the incidence of root diseases caused by these pathogens.As for antagonistic bacteria, [9] found that seed treatment with Bacillus spp. was actively controlled three fungal root diseases of wheat. Also, Pseudomonas cepacia or Pseudomonas fluorescens applied to pea seeds act as biological control agent against Pythium damping-off and Aphanomyces root rot and was able to reduce diseases incidence [10,11]. Considerable researches has been done to investigate antagonistic microbes for use in seed treatments as reported by several workers[12-15]. In this regard, many crops are susceptible to seed and seedling root rot caused by soilborne fungi or by pathogens carried on the seed. Biological seed treatments may provide an alternative to chemical control of many soil and seed-borne pathogens. Bio-priming, a seed treatment system that integrates the biological and physiological aspects of disease control, involves coating the seed with fungal or bacterial biocontrol agents. Furthemore, soil drench with T. harzianum was significantly able to reduce the incidence of bean root rot of bean and pepper wilt diseases [2,16].The objective of the present study was to evaluate the different approaches activity of some antagonistic fungal and bacterial agents against vegetables root rot incidence when applied as seed treatment or soil drench under open greenhouse conditions.
2. Materials and Methods
2.1. Plant Materials
- Seeds of Cucumber (cv. Alpha), Cantaloupe (cv. Yatherb 7), Tomato (cv. Castel Rock) and Pepper (cv. California) were used in the present study.
2.2. Pathogenic Fungi
- The tested soilborne pathogenic fungi were Alternaria solani Fusarium solani, F. oxysporum, Rhizoctonia solani, Sclerotium rolfsii, Macrophomina phaseolina and Pythium sp. These fungi were isolated from various vegetables, i.e. Cucumber, Cantaloupe, Tomato and Pepper grown in plastic houses under protected cultivation system and showing root rot and or damping-off disease symptoms[17]. The isolated fungi proved their aggressive ability to induce root rot disease of those vegetables.
2.3. Bio-agents
- The tested antagonistic fungi were Trichoderma harzianum, T. Viride and Bacillus subtilis, Pseudomonas flourescens and Sacchromyces serivisae. These antagonists were isolated from cucumber, cantaloupe, tomato and pepper grown in plastic houses under protected cultivation systems and showing root rot disease symptoms[17]. The present bio-agents proved their antagonistic ability against the above mentioned pathogens under in vitro conditions.
2.4. Bio-agents and Pathogens Inocula Preparation
- The antagonistic bacteria (B. subtilis, P. flourescense) were grown on nutrient broth medium while yeast (S. serevisiae) was grown on NYDB medium[18]. All tested bacteria and yeast incubated in a rotary shaker at 200 rpm for 24 h at 28 ±2℃. The bacterial and yeast cells were harvested by centrifugation at 6,000 rpm for 10 min, washed twice with 0.05 M phosphate buffer at pH 7.0, and re- suspended in sterilized distilled water. The concentrations of both bacteria and yeast cells in the suspensions were adjusted to 1x105-106 cells per milliliter (Cfu / mL) with the aid of a haemacytometer slide. Meanwhile, antagonistic fungi were grown on PDA medium[18] incubated for 72 h at 25 ±2℃. Fungal conidia were harvested by scraping the surface of the colonies with a spatula and transferred to sterilized distilled water and filtered through nylon mesh, then spore suspension was adjusted 1x104-105 spore per milliliter (Cfu / mL) with the aid of a haemacytometer slide.On the other hand, Inocula of pathogenic fungi, Alternaria solani Fusarium solani, F. oxysporum, Rhizoctonia solani, Sclerotium rolfsii, Macrophomina phaseolina and Pythium sp. were individually grown on autoclaved sand barlly medium (1:1,v:v + 40% water) for two weeks at 25±1℃ (Abdel-Kader, 1997). Soil infestation was carried out through amended individually with a pathogenic fungal inoculum (5% w:w) after[16], then mixed thoroughly to ensure equal distribution of the added inoculum. Infested soil was then filled in plastic pots (30-cm-diameter) and irrigated every second day for 1 week before sowing.
2.5. Greenhouse Experiments
- Evaluation the efficacy of different approaches of bio- agents against root rot incidence of Cucumber, Cantaloupe, Tomato and Pepper was carried out in pot experiment in the open greenhouse of Plant Pathology Dept., National Research Centre, Egypt. The evaluated bio-agents were applied either as seed soaking or soil drench.Pathogens infested soil with the root rot pathogens inocula was used for this experiment. Seeds of Cucumber, Cantaloupe, Tomato and Pepper were soaked in individual fungal, bacterial or yeast sticky suspensions (1 mL of Arabic gum/100 suspension) for one hr, then picked up and air dried. Soaked seeds were sown as five seeds per pot, six pots per replicates in each treatment.As for soil drench, the pathogens infested soils were re-infested individually with inocula of bio-agents either fungi (Trichoderma harzianum, T. viride, T. hamatum); bacteria (Bacillus subtilis, Pseudomonas fluorescence) or yeast (Saccharomyces serevisiae) relevant to the specific treatment. The fungal inocula were added to the soil at the ratio of 5% of soil weight, while bacterial inocula as liquid cultures (3x106 cfu/mL) at the ratio of 100 ml/cubic foot of soil.Another set of varied infested soils only with pathogens were filled in plastic pots (30- cm-diameter), was used for comparison check treatment. Another set of soil amended only with root rot pathogens (5% w:w) was Kept as control check treatment.Surface sterilized seeds (using 3% sodium hypochlorite for 5 min, then picked up and air-dried) were used. Five seeds of each of Cucumber, Cantaloupe, Tomato and Pepper were planted in each pot and six replicated pots were used for each particular treatment. Percentage of root rot incidence at pre-emergence growth stage was recorded after two weeks from sowing date. Meanwhile, percentage of root rot incidence at post-emergence stage was calculated 45 days after seedling emergence. This experiment was repeated twice in order to confirm the obtained results.Percentage of root rot incidence at the pre-emergence stage was calculated as the number of absent seedlings relative to the number of seeds sown. Meanwhile, the percentage of root rot incidence at the post-emergence stage was calculated as the number of diseased plants relative to the number of emerging seedlings. At the end of the experiment, survived plants were carefully pulled out from pots after being flooded with water in order to leave the root system undamaged. Plant roots showing rot lesions in addition to the visual root rot symptoms on the shoot system were considered diseased plants. Isolation from infected germinated seeds at the pre-emergence stage and infected plants at the post-emergence stage was carried out. Undeveloped, germinated seeds which were picked up from the soil, and the diseased plants were both washed and sterilized with 3% sodium hypochlorite, then subjected to re-isolation in order to identify the causal pathogens.Statistical analysis
3. Results and Discussion
|
|
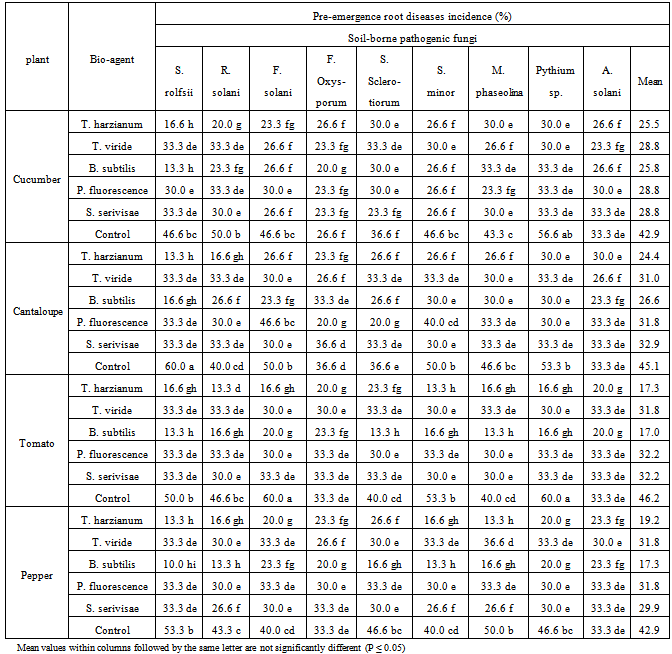
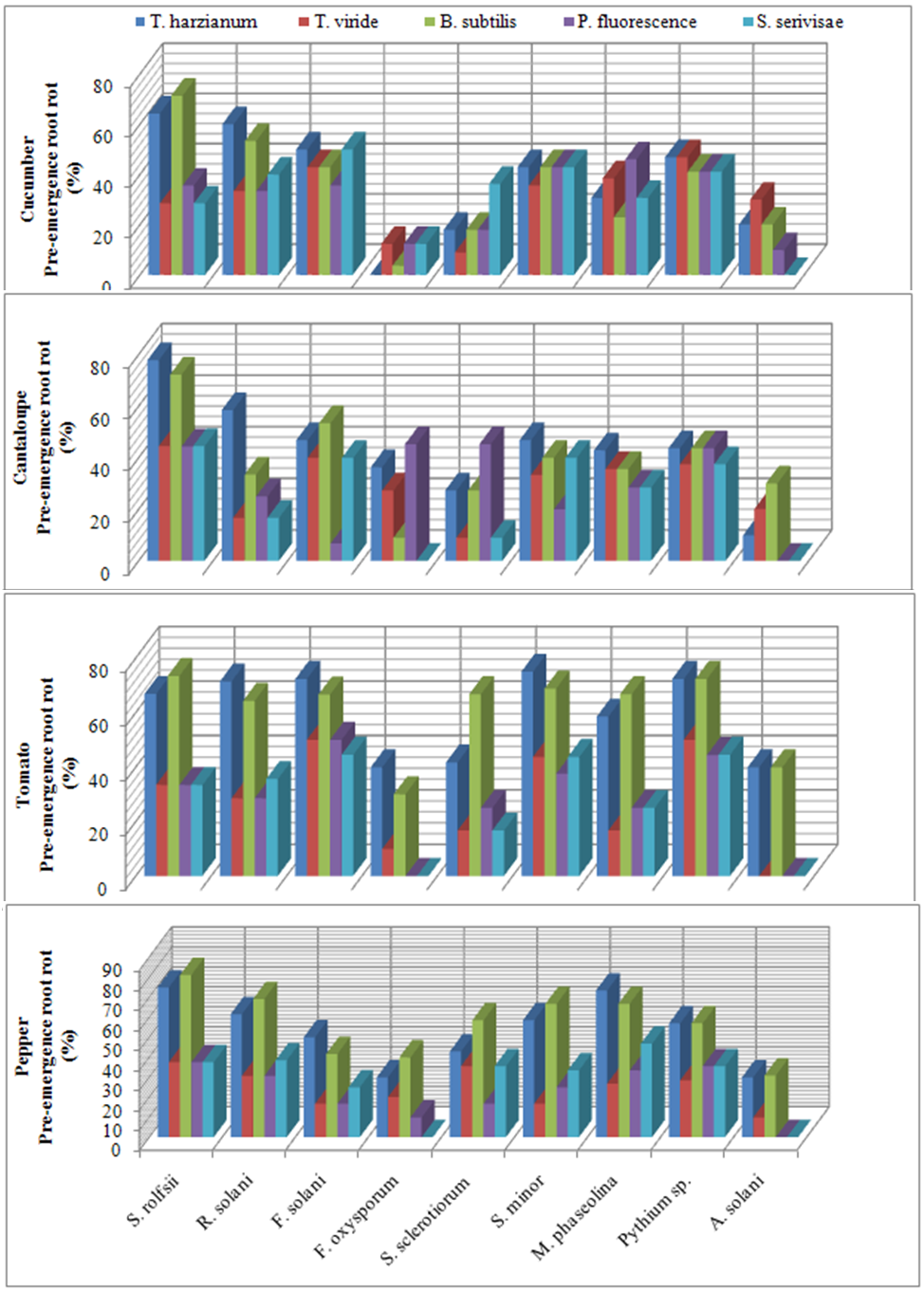 | Figure 1. Reduction in vegetables root diseases vegetables root diseases at pre-emergence growth stage caused by soil-borne pathogenic fungi in response to applying antagonistic bio-agents as seed dressing under open greenhouse conditions |
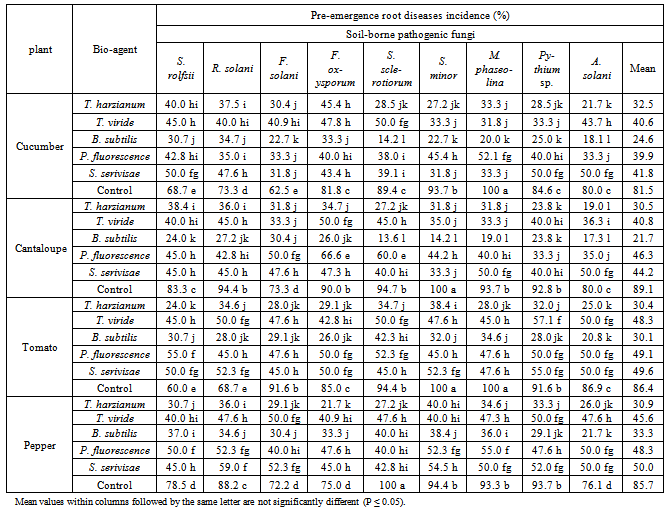
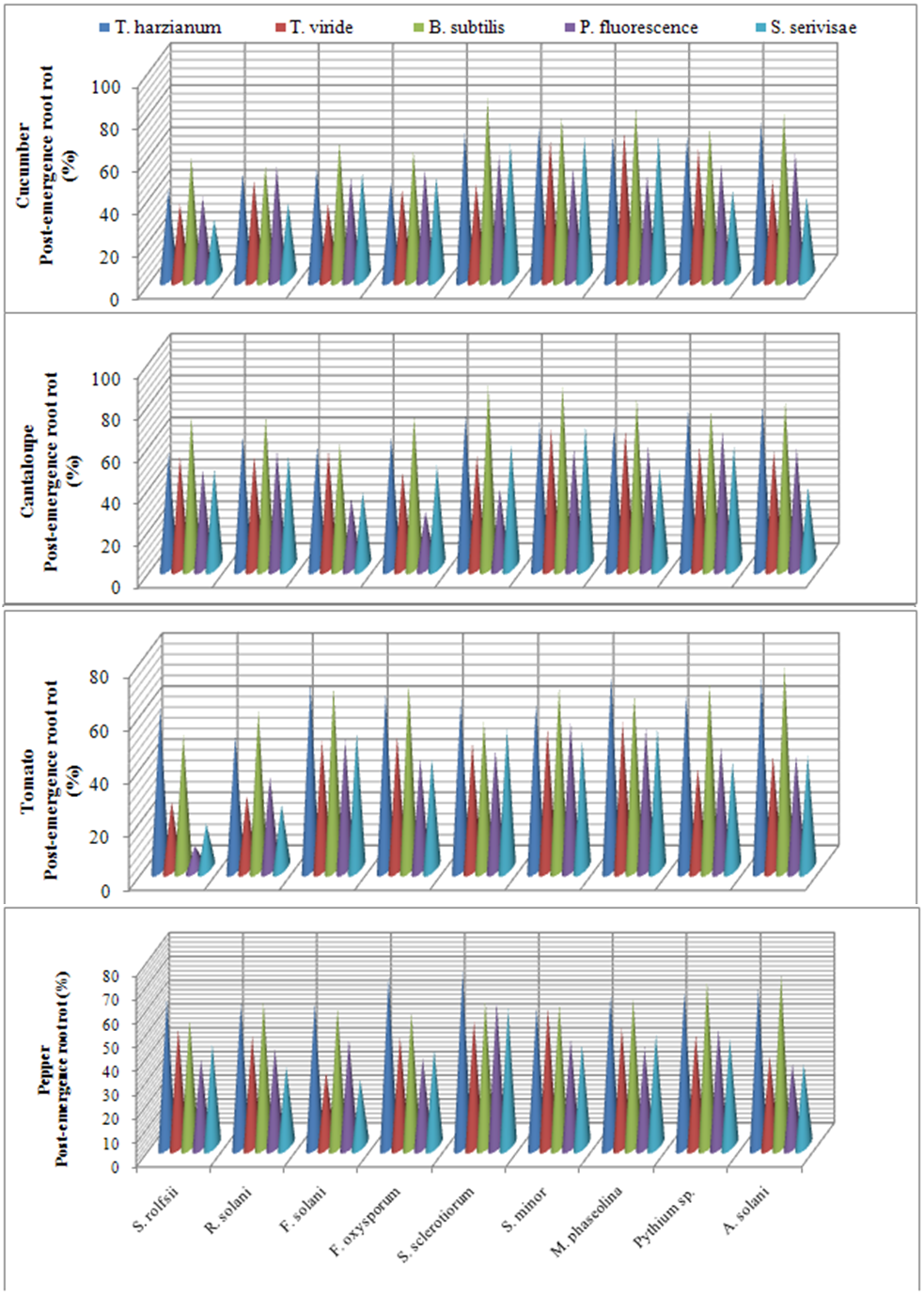 | Figure 2. Reduction in vegetables root diseases vegetables root diseases at post-emergence growth stage caused by soil-borne pathogenic fungi in response to applying antagonistic bio-agents as seed dressing under open greenhouse conditions |
|
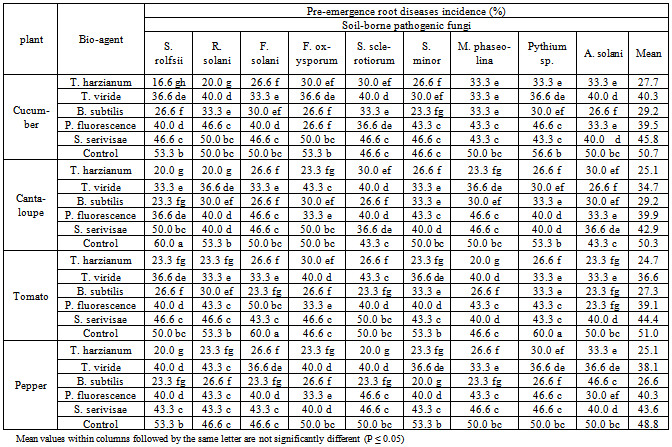
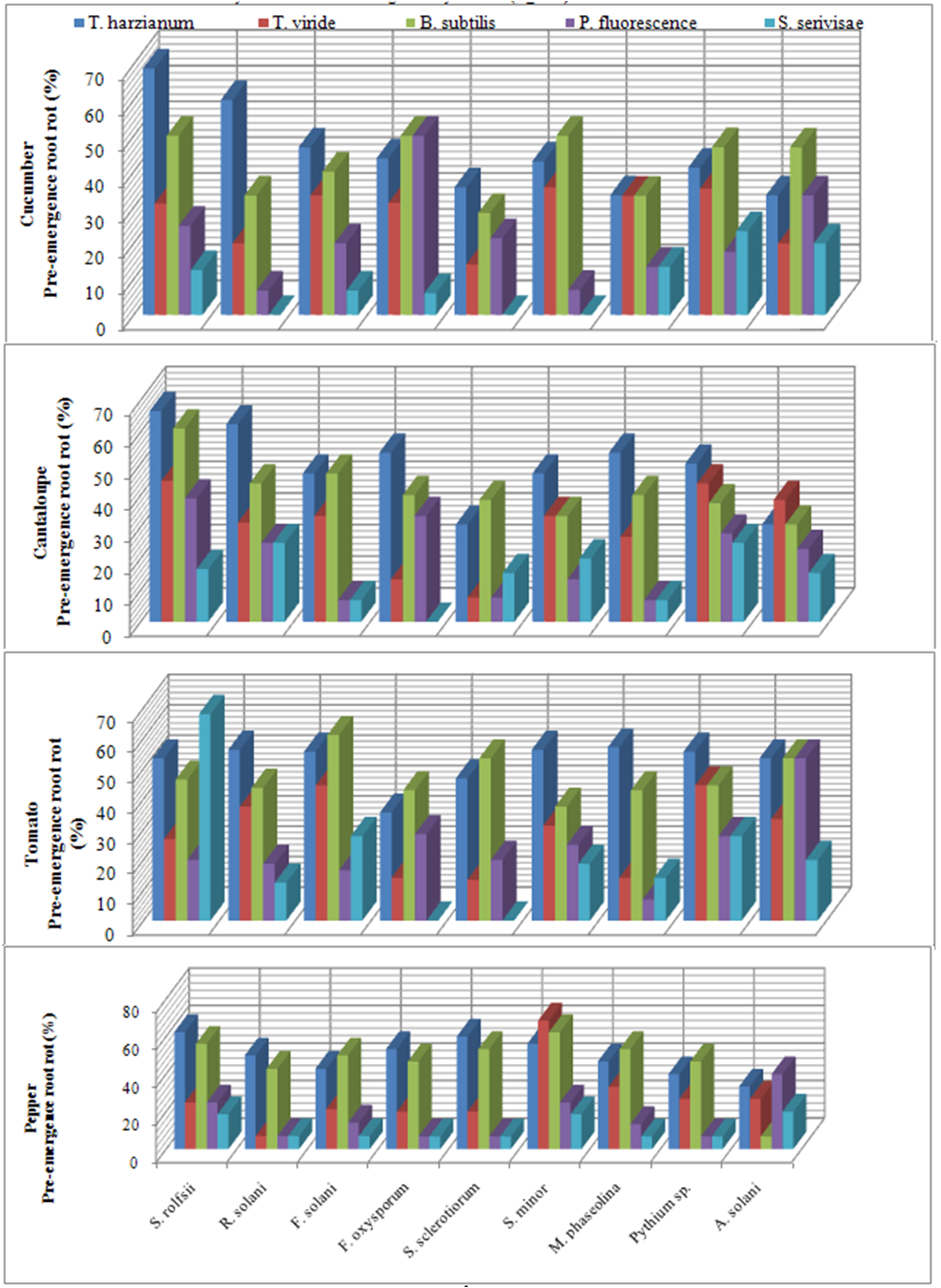 | Figure 3. Reduction in vegetables root diseases vegetables root diseases at pre-emergence growth stage caused by soil-borne pathogenic fungi in response to applying antagonistic bio-agents as soil treatment under open greenhouse conditions |
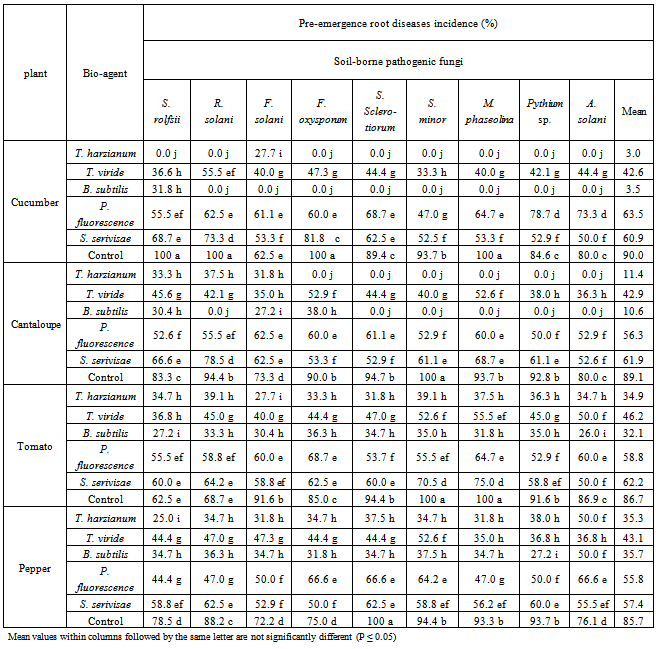
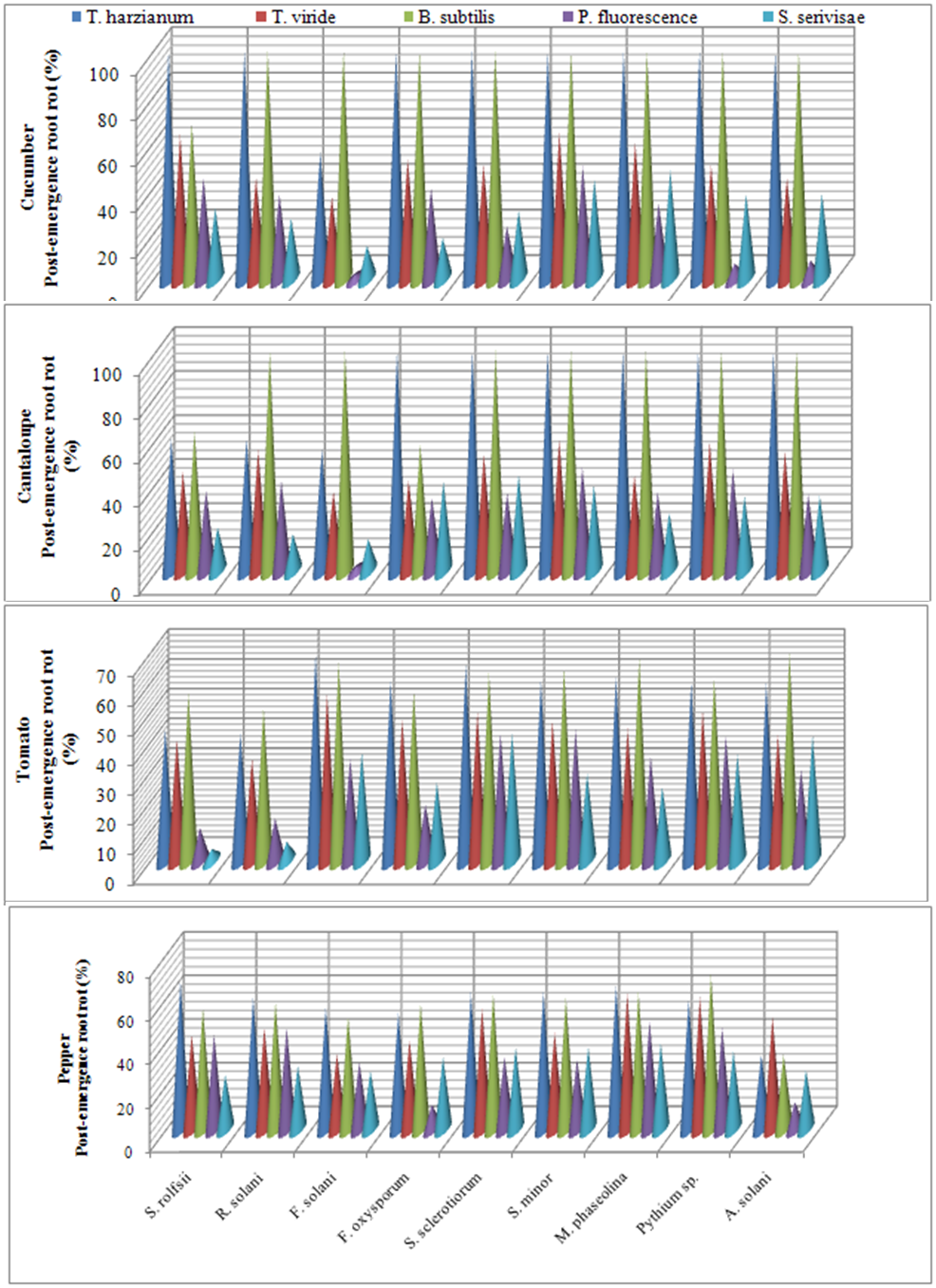 | Figure 4. Reduction in vegetables root diseases vegetables root diseases at post-emergence growth stage caused by soil-borne pathogenic fungi in response to applying antagonistic bio-agents as soil treatment under open greenhouse conditions |
ACKNOLEDGMENTS
- This work was supported financially by the Science and Technology Development Fund (STDF), Egypt, Grant No. 1059.
References
| [1] | Sivan, A., 1987, Biological control of Fusarium crown rot of tomato by Trichoderma harzianum under field conditions. Plant Dis., 71, 587–592. Doi: 10.1094/PD-71-0587. |
| [2] | Abdel-Kader, M.M., 1997, Field application of Trichoderma harzianum as biocide for control of bean root rot disease. Egypt. J. Phytopathol., 25, 19–25. |
| [3] | Sivan, A., Chet, I., 1986, Biological control of Fusarium spp. in cotton, wheat and muskmelon by Trichoderma harzianum. J. Phytopathol., 116, 39–47. Doi: 10.1111/j.1439-0434.1986. tb00892.x. |
| [4] | Whipps, J.M., Lumsden, R.D., 2001, Commercial use of fungi as plant disease biological control agents: status and prospects. p. 9–22. In: “Fungi as Biocontrol Agents: Progress, Problems and Potential” (T.M. Butt, C. Jackson, N. Magan, eds.), (2001), CABI Publishing: Wallingford, UK. |
| [5] | McLean, K.L., Dodd, S.L., Sleight, B.E., Hill, R.A., Stewart, A., 2004, Comparison of the behavior of a transformed hygromycin resistant strain of Trichoderma atoviride with the wild-type strain. NZ Plant Protect., 57, 72–76. |
| [6] | Hadar, Y., Chet, I., Henis, Y., 1979, Biological control of R. solani damping-off with wheat bran culture of Trichoderma harzianum. Phytopathology, 69, 64–68. |
| [7] | Hadar, Y., Harman, G.E., Taylor, A.G., 1984, Evaluation of Trichoderma koningii and T. harzianum from New York soils for biological control of seed rot caused by Pythium spp. Phytopathology, 74, 106–110. |
| [8] | Elad, T., Chet, J., Katan, J., 1980, Trichoderma harzianum: a biocontrol effective against Sclerotium rolfsii and Rhizoctonia solani. Phytopathology, 70, 119–121. |
| [9] | Kim, D.S., Cook, R.J., Weller, D.M. 1997, Bacillus sp. L324-92 for biological control of three root diseases of wheat grown with reduced tillage. Phytopathology, 87,551-558. |
| [10] | Parke, J.L., Rand, R.E., Joy, A.E., King, E.B., 1991, Biological control of Pythium damping-off and Aphanomyces root rot of peas by application of Pseudomonas cepacia or P. fluorescens to seed. Plant Disease, 75,987-992. |
| [11] | King, E.B., Parke, J.L., 1993. Biocontrol of Aphanomyces root rot and Pythium damping–off by Pseudomonas cepacia AMMD on four pea cultivars. Plant Disease, 77, 1185-1188. |
| [12] | Callan, N.W., Mathre, D.E., Miller, J.B., 1990, Bio-priming seed treatment for biological control of Pythium ultimum pre-emergence damping-off in sh2 sweet corn. Plant Disease, 74, 368-372. |
| [13] | Baird, R.E., Nankam, C., Moghaddam, P.F., Pataky, J., 1994,. Evaluation of seed treatments on Shrunken-2 sweet corn. Plant Disease, 78, 817-821. |
| [14] | Howell, C.R., Stepanovic, R.D., 1995,. Michanism in the biocontrol of Rhizoctonia solani – induced cotton seedling disease by Gliocladium virens: Antibiosis. Phytopathology, 85, 469-472. |
| [15] | Mathre, D.E., Johnston, R.H., Callan, N.W., Mohan, S.K., Martin, J.M., Miller, J.B., 1995, Combined biological and chemical seed treatments for control of two seedling diseases of sh2 sweet corn, Plant Disease, 79, 1145-1148 |
| [16] | Abdel-Kader, M.M., 1999, Biological and chemical control of wilt disease of hot pepper (Capsicum annum L.). Egypt. J. Phytopathol., 27, 1-8. |
| [17] | El-Mougy, N.S., Abdel-Kader, M.M., Abdel-Kareem, F., Embaby, E.I., El-Mohamady, R., Abd El-Khair, H., 2011, Survey of Fungal Diseases Affecting Some Vegetable Crops and Their Rhizospheric Soilborne Microorganisms Grown under Protected Cultivation System in Egypt. Research Journal of Agriculture and Biological Sciences, 7,(2), 203-211. |
| [18] | Abd-Alla, M.A., El-Mohamedy, R.S.R., El-Mougy, N.S., 2007, Control of Sour Rot Disease of Lime Fruits Using Saprophytic Isolates of Yeast. Egypt. J. Phytopathol., 35, (2),_ 39-51. |
| [19] | SAS, Institute Inc., ‘SAS/STAT user’s guide. Version 6. Vol. 2.’ 12th edn. (SAS Institute Inc.: Cary, NC), (1996), 846 pp. |
| [20] | Winer, B.J., ‘Statistical principles in experimental design.’ 2nd edn. (McGraw-Hill Kogakusha Ltd: Tokyo), (1971), 596 pp. |
| [21] | Papavizas, G.C., 1982, Survival of Trichoderma harzianum in soil and in pea and bean rhizospheres. Phytopathology, 72, 121-125. |
| [22] | Monte, E., 2001, Understanding Trichoderma: between biotechnology and microbial ecology. Int. Microbiol., 4,1- 4. |
| [23] | Chao W.L., Nelson E.B., Harman G.E., Hoch H.C. 1986. Colonization of the rhizosphere by biological control agents applied to seeds. Phytopathology 76: 60–65. |
| [24] | El-Mougy, N.S., 2001, Field application of certain biological and chemical approaches on controlling Bean wilt disease. Egypt. J. Phytopathol., 29, 69–78. |
| [25] | Wright, B., Rowse, H.R., Whipps, J.M., 2003, Application of beneficial microorganisms to seeds during drum priming. Biocontrol Sci. Technol., 13, 599–614. Doi:10.1080/09583150310001517992 |
| [26] | El-Mougy, N.S., Abdel-Kader, M.M., 2008, Long term activity of bio-priming seed treatment for biological control of faba bean root rot pathogens. Aust. Plant Pathol., 37, (5), 464–471. |
| [27] | Baker, K.F., Cook, R.J., 1974, Biological control of plant pathogens. W. H. Freeman & Co., San Francisco, CA, USA., (1974). |
| [28] | Ahn, S.J., Hwang, B.K., 1992, Isolation of antibiotic- producing actinomycetes antagonistic to Phytophthora capsici from pepper-growing soils. Korean J. Mycol., 20,259-268. |
| [29] | Jee, H.J., Nam, C.G., Kim, C.H., 1988, Studies on biological control of Phytophthora blight of red-pepper. I. Isolation of antagonists and evaluation of antagonistic activity in vitro and in greenhouse. Korean J. Plant Pathol., 4,305-312. |
| [30] | Kim, B.S., Hwang, B.K., 1992, Isolation of antibiotic- producing bacteria antagonistic to Phytophthora capsici from pepper growing soils and evaluation of their antibiotic activity. Korean J. Plant Pathol., 8,241-248. |
| [31] | Park, K.S., Jang, S.W., Kim, C.H., Lee, E.J., 1989, Studies on biological control of Phytophthora blight of red-pepper. III. Formulations of Trichoderma harzianum and Pseudomonas cepacia antagonistic to Phytophthora capsici and their preservation. Korean J. Plant Pathol., 5,131-138. |
| [32] | Gomathinayagam, S., Rekha, M., Murugan, S., Jagessar, J.C., 2010, The biological control of paddy disease brown spot (Bipolaris oryzae) by using Trichoderma viride in vitro condition. Journal of Bio. pesticides, 3, 93-95. |
| [33] | Peighami-Ashnaei, S., Sharifi-Tehrani, A., Ahmadzadeh, M., Behboudi, K., 2009, Interaction of different media on production and biocontrol efficacy of Pseudomonas fluorescens P-35 and Bacillus subtilis B-3 against grey mould of apple. Journal of Plant Pathology, 91, (1), 65-70. |
| [34] | Ahimou, F., Jacques, P., Deleu, M., 2000, Surfactin and iturin A effects on Bacillus subtilis surface hydrophobicity. Enz. Microb. Technol., 27, 749-752. |
| [35] | Worthington, P.A., 1988, Antibiotics with antifungal and antibacterial activity against plant diseases. Nat. Prod. Rep., 5, 47-50. |
| [36] | Podile, A.R., Parkash, A.P., 1996, Lysis and biological con trol of Aspergillus niger by Bacillus subtilis AF 1. Can. J. Microbiol., 42, 533-537. |
| [37] | Bull, C.T., Stack, J.P., Smilanick, J.L., 1997, Pseudomonas syringae strains ESC-10 and ESC-11 survive in wounds on citrus and control green and blue molds of citrus. Biol. Control, 8, 81-88. |
| [38] | Janisiewicz, W., Yourman, L., Roitman, J., Mahoney, N., 1991, Postharvest control of blue mold and gray mold of apples and pears by dip treatment with pyrrolnitrin, a metabolite of Pseudomonas cepacia. Plant Dis., 75, 490-494. |
| [39] | El-Ghaouth, A., Wilson, C. L., Wisniewski, M., 1998, Ultrastructural and cytochemical aspects of the biological control of Botrytis cinerea by Candida saitoana in apple fruit. Phytopathology, 88, 282-291. |
| [40] | Wisniewski, M., Biles, C., Droby, S., McLaughlin, R., Wilson, C., Chalutz, E., 1991, Mode of action of the postharvest biocontrol yeast, Pichia guilliermondii. I. Characterization of the attachment to Botrytis cinerea. Physiol. Mol. Plant Pathol., 39, 245-258. |
| [41] | Agrios, G.N., 1997. Plant Pathology, 4th edn, Academic Press, (1997). |
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML