-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Health Science
p-ISSN: 2166-5966 e-ISSN: 2166-5990
2022; 12(1): 1-6
doi:10.5923/j.health.20221201.01
Received: Dec. 3, 2021; Accepted: Dec. 17, 2021; Published: Jan. 13, 2022

Occupational Exposure to Bisphenol A (BPA) is a Risk Factor for Coronary Heart Disease
Tunmise Tope Oladipe1, 2, Patrick Ojeifor Uadia2
1Department of Chemical Sciences, College of Natural and Applied Sciences, Salem University Lokoja, Kogi State, Nigeria
2Department of Biochemistry, Faculty of Life Sciences, University of Benin, Benin City, Nigeria
Correspondence to: Tunmise Tope Oladipe, Department of Chemical Sciences, College of Natural and Applied Sciences, Salem University Lokoja, Kogi State, Nigeria.
| Email: |  |
Copyright © 2022 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Bisphenol A (BPA) is one of the endocrine disrupting chemicals, to which humans are continuously exposed. Most studies that have reported its human health hazards have focused on the general population, and only a few studies have associated occupational exposures to BPA with adverse health outcomes. This study compared the association between serum concentrations of BPA in occupationally and non-occupationally exposed adults with their lipid profiles. Serum samples obtained from occupationally exposed adults (n=46) and non-occupationally exposed adults (n=23), were analysed for BPA, using enzyme linked immunosorbent assay (ELISA) method, serum triglyceride (TG), total cholesterol (TC), high density lipoprotein cholesterol (HDL-c), low density lipoprotein cholesterol (LDL-c), atherogenic index (AI) Body Mass Index (BMI) were also determined. The result revealed a statistically non-significant higher mean serum BPA concentration in the occupationally (BPA) exposed group (3.30±1.46ng/ml) compared with the non-occupationally exposed group (0.05±0.01ng/ml). No significant differences were found between the occupationally exposed group and the control group for serum TC, TGs, LDL-c, AI, malondialdehyde (MDA) and body mass index (BMI). However there was a significantly lower serum HDL-c (p=0.003) in the occupationally exposed group compared with the non-occupationally exposed control group. In addition there was a statistically significant association between longer duration of exposure to BPA and higher serum TC and AI among the occupationally exposed individuals. In conclusion the study revealed that a higher serum BPA level is associated with lower serum HDL-c among occupationally exposed individuals.
Keywords: Occupational exposure, Bisphenol A (BPA), Serum levels, Lipid profiles
Cite this paper: Tunmise Tope Oladipe, Patrick Ojeifor Uadia, Occupational Exposure to Bisphenol A (BPA) is a Risk Factor for Coronary Heart Disease, Journal of Health Science, Vol. 12 No. 1, 2022, pp. 1-6. doi: 10.5923/j.health.20221201.01.
Article Outline
1. Introduction
- BPA is estrogenic [1], because of its estrogenic properties in vitro and in vivo and the conserved role that estrogen plays in regulating human and animal physiology and pathophysiology [1,2]. Data from multiple sources indicated that the amount of BPA to which humans are exposed may cause adverse health effects; this has raised concerns among regulatory agencies all over the world. In developed countries exposure to BPA is significant and continuous. Indeed, it has been suggested that exposure to xenoestrogens such as BPA during early development may be a major contributing factor to the increased incidence of infertility, genital tract abnormalities, obesity, attention deficit hyperactivity disorder (ADH), prostate and breast cancer observed in European and U.S. human populations over the last 50 years [3]. In a study by Hong et al, BPA concentrations in urine and blood collected from 516 Korean were associated with at least one marker of oxidative stress [4], another study by Yang et al. [5] found that total BPA levels were associated with markers of stress in postmenopausal women, but not in premenopausal women or men, leading the authors to suggest that postmenopausal women may be more susceptible to BPA-induced adverse health effects. Several smaller studies have examined the effects of BPA exposure on other health outcomes. For instance, BPA levels in blood have been associated with a variety of conditions in women, including obesity, endometrial hyperplasia, endometriosis, recurrent miscarriages, sterility, and polycystic ovary syndrome (PCOS) [6], while other studies detected associations between high BPA exposure and chromosomal abnormalities, including pregnancies with foetuses that had an abnormal karyotype [7], recurrent miscarriage [8], and sister chromatid exchange measured in peripheral lymphocytes [9]. Other studies have identified relationships between BPA exposure and reproductive hormone levels in male patients at an infertility clinic [10] and the number of oocytes retrieved from women undergoing in vitro fertilization (IVF) fertility treatments [11]. Most of the human health hazards reports on BPA were obtained from non-occupationally exposed individuals, and thus a data gap exists in relation to exposure levels in employees in BPA-producing industries or those workers using BPA in different manufacturing processes. Thus studies focusing on occupational exposure to BPA and its health effects are therefore needed; this study compared the health effects of occupational and environmental exposure to BPA using volunteers from South-Western Nigeria.
2. Materials and Methods
2.1. Subjects
- The subjects consisted of 46 volunteer workers (male and female) at a plastic manufacturing industry in Ibadan, Oyo State Nigeria. They had spent over 10 years or less in the industry, and were aged between 18 and 60 years. Also, 23 age-matched apparently healthy non-plastic workers were used as control subjects. All participants signed an informed consent form and a well-explained questionnaire was distributed to each of them. Only those adults who returned their questionnaire were enlisted for the study. The questionnaire used was a structured type of questionnaire (fixed response format) containing information that actually helped in the stratification of the sample population. The study was presented to all participating factory workers as a study of health effects of general occupational hazard. Therefore, all participants were blinded to the specific hypothesis related to the effect of BPA.The contents of the questionnaire included name, age, sex, period of time spent in the industry, department in the industry, if there is any protective personal clothing or uniform, marital status, number of children, whether or not there was difficulty in conception, any health challenges. The study was performed in February 2016 at Ibadan and Owo town, in South-West Nigeria. Approval for the research was given by the ethics committee of the Federal Medical Centre (FMC) Owo, Ondo State, Nigeria.
2.2. Sample Collection
- Fasting venous blood samples were drawn between 8 a.m. to 11 a.m., into fluoride oxalate bottle for the determination of plasma glucose and into plain bottles to obtain serum for hormone analysis and analysis of clinical parameters. The plasma and serum were centrifuged and separated within 30 minutes of collection and frozen at −20°C within ten hours. All samples were taken to the laboratory on dry ice and analyzed for hormone concentrations and clinical parameters.
2.3. Biochemical Analysis
2.3.1. Determination of Serum BPA Levels of Subjects
- Serum BPA was analyzed by enzyme-linked immunosorbent assay (ELISA) method using BPA ELISA kit from Detroit R&D, Inc, Metro Centre for High Technology Bldg. (MCHT) Detroit, USA, with product code Cat # BPA 1.
2.3.2. Determination of Serum Triglyceride, Total Cholesterol and HDL-cholesterol
- Serum triglyceride, total cholesterol and HDL-cholesterol were measured by the quantitative enzymatic colorimetric technique as described in using kits supplied by RANDOX laboratories limited, UK.
2.3.3. Serum LDL-cholesterol and Atherogenic Index (AI)
- Serum LDL-cholesterol and Atherogenic index (AI) were calculated according to Friedewald et al. [12] and Grundy et al. [13] respectively as follows:LDL-c mg/dl = Total cholesterol – (triglyceride/5 + HDL-c)Atherogenic index =
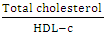
2.3.4. Calculation of Body Mass Index (BMI)
- Body mass index was calculated according to the formula: BMI = weight (kg) / height (m2) kg/m2 [14]
2.3.5. Determination of Serum Malondialdehyde (MDA) Level
- Malondialdehyde (MDA) a product of lipid peroxidation in the serum was assayed using the method the method of Hunter et al. [15] modified by Gutteridge and Wilkins [16]. 0.2mls of the serum was treated with 1ml each of Glacial acetic acid and 1% TBA, placed in a water bath at 37°C for 15 min, cooled and centrifuged. The absorbance of the clear supernatant was measured against reference blank at 532nm.
2.4. Statistical Analysis
- Values were expressed as the mean ± SEM. Results were statistically analyzed by one-way analysis of variance (ANOVA) for differences between means of different groups. All data were analyzed using SPSS statistical package (SPSS Inc.). A probability of P<0.05 was considered statistically significant.
3. Results
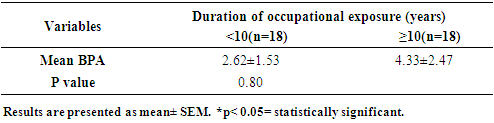
- Among the 46 occupationally exposed individuals who provided blood samples, total BPA was detected in 80% (36) with a mean concentration of 3.30ng/ml BPA while among the non-occupationally (control) exposed group (n=23) total BPA was detected in only 65% (15) of the volunteers with a mean concentration of 0.05ng/ml BPA. Table 1 shows the serum mean BPA concentration in both occupationally exposed and the control group. The serum BPA concentration between the occupationally exposed group and the control group was statistically non-significant (p=0.2).However when the result was adjusted for duration of occupational exposure at the plastic industry as shown in table 2, the results showed that those who had worked in the plastic factory for over 10 years had higher serum BPA level compared with those who worked for less than 10 years at the factory. However the difference in the serum BPA was statistically non-significant (p=0.8).
|
|
|
|
4. Discussion
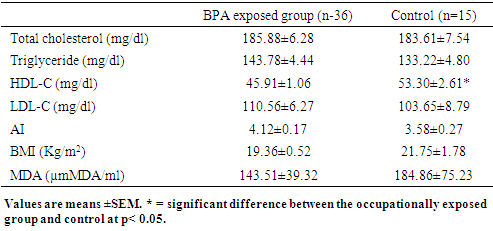
- Most adverse health effect study of BPA has been on the general population, this limitation stems from the belief that BPA exposure comes predominantly from contamination via food sources [17]. Despite reports suggesting exposure of healthy individuals to BPA by non-oral routes [18,19], hence there is a data gap relative to exposure levels in employees in BPA-producing industries or workers using BPA in different manufacturing processes. In addition BPA has been measured in human urine from several populations around the world. Hence the adverse health effects associated with BPA is due to its long term health effects which are most likely associated with long-term, low-dose exposure. Hence there is need for studies evaluating serum BPA levels in humans to determine the level of internal exposure to unconjugated BPA and its health implication. This present study therefore evaluated the association between circulating levels of BPA and health in non-occupationally exposed and occupationally exposed individuals. In this present study circulating BPA was detected in only 75% of the participants. Circulating BPA concentrations can differ for systemic reasons, such as differences in sample collection, with a mean concentration of 3.30±ng/ml, while among the non-occupationally exposed group total BPA was detected in only 65% of the participants with a mean concentration of 0.05±ng/ml. The mean serum BPA concentration of the occupationally exposed volunteers was 66 folds higher than the mean serum BPA concentration of non-occupationally participants, although the difference is statistically non-significant (p>0.05). This may be due to the wide range of circulating BPA found among the occupationally exposed group due to additional exposure to BPA via other sources of human exposure (exposure in air and dust). Several studies measuring exposure in air and dust [20,21,22], have documented the additional importance of exposures that avoid the first-pass hepatic metabolism following oral exposure. In addition, faster metabolic rate of BPA (within hours) [23], or differences in the rate of BPA metabolism among participants is also an important factor. BPA is non-persistent in the body; they metabolize quickly and, as a result, the levels of BPA in participants may be affected by other factors such as, body size, genetics, circulating levels of other hormones or metabolites, time of exposure, haematological parameters and nutrition. Hence serum samples collected may reflect only very recent exposures and cannot characterize average BPA exposures for individuals. The BPA levels obtained in the present study are comparable with those from several previous studies, several of which consist of women with different endocrinological problems and their controls [24,25,26,27,28]. Some studies show lower values of BPA in human serum [29,30,31]. While another study found higher circulating BPA, for previous studies the mean circulating BPA concentration determined by ELISA was 12.0 ng/mL (9.1μg/g Creatinine).The unexposed group in our study also had detectable serum BPA level, it is well recognized that ingestion is the most frequent route of BPA exposure in non-occupationally exposed population, while the occupationally exposed population may be exposed to BPA from additional routes of dermal, inhalation, and ingestion through exposure in the workplace [17,19]. The lower serum BPA level found in the non-occupationally exposed group may be due to the rapid metabolism of BPA that occur after oral exposure [32]. This study also revealed that there is no significant association between duration of occupational exposure to BPA and serum BPA level, this can be explained by the fact that BPA is rapidly metabolized in humans via hepatic glucuronidation and sulfation, and the major metabolite BPA-G (water soluble) is rapidly excreted through the kidneys with half-life of approximately 6 hrs and complete urinary excretion in 24 hr [23]. Hence the long-term effects associated with BPA exposure in this group may results from a low-dose continuous exposure. The importance of HDL-cholesterol as a risk factor for coronary heart disease is now recognized. Elevated circulating levels of environmental contaminants and pollutants have been found to be associated with prevalent coronary heart disease (CHD), a number of cardiovascular (CV) risk factors such as hypertension, obesity, and diabetes, and metabolic syndrome [33,34]. Urinary levels of BPA have been found to be associated with CHD in the NHANES sample [35,36]. There is also another study suggesting that exposure to BPA and phthalates, measured in serum, is associated with atherosclerosis [37] (Lind and Lind, 2011). Table 3, shows the association between serum BPA of participants (occupationally exposed and control) and lipid profile. In the current study, although there were no significant (P>0.05) differences between the occupationally exposed group and control for total cholesterol, triglycerides, LDL-C, atherogenic index (AI) and body mass index (BMI), the occupationally exposed group had a significantly (P<0.003) lower HDL-C level compared with the non-occupationally exposed group. The non-significant differences in the total cholesterol, AI, LDL-C and BMI between the BPA exposed group and the control may be due to different metabolic rates among the exposed individuals. Although a similar study by Hussein et al. [38] did not find any significant difference in the mean age, BMI and work duration between those who are occupationally exposed to BPA and control group, in this present study, serum total cholesterol, LDL-C and atherogenic index was significantly (p<0.05) higher for those who had spent ≥ 10 years in the plastic factory compared with those who had spent <10 years.
5. Conclusions
- In conclusion, this study revealed that the workers exposed to BPA in the plastic manufacturing industry have increased level of serum BPA compared with the non- occupationally exposed (control) group. Also, the increased BPA in the occupationally exposed individuals is associated with lower HDL-C. The present study added to the evidence that BPA is disruptive to the endocrine system and hence human’s hormone homeostasis. Together, these data is in support of concerns about the potentially deleterious impact of BPA on human health. Public awareness of these deleterious effects should be strengthened and protection against BPA exposure should be promoted. Therefore, environmental monitoring of BPA in the workplace is important and the use of protective clothing should be encouraged, because inhalation of BPA dusts particles and through dermal route is the major route of occupational exposure.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML