-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Health Science
p-ISSN: 2166-5966 e-ISSN: 2166-5990
2017; 7(1): 1-6
doi:10.5923/j.health.20170701.01

Anti-Measles Virus (MV) IgM Antibodies in Unvaccinated Children Population in Emohua, Rivers State, Nigeria
Okonko Iheanyi Omezuruike, Jim-George Onari
Medical Microbiology Unit, Department of Microbiology, University of Port Harcourt, Port Harcourt, Nigeria
Correspondence to: Okonko Iheanyi Omezuruike, Medical Microbiology Unit, Department of Microbiology, University of Port Harcourt, Port Harcourt, Nigeria.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The study sets to ascertain the seropositivity of anti-measles virus (MV)-specific IgM antibodies in unvaccinated children population of Emohua, Rivers State, Nigeria. Measles virus-specific IgM antibodies was examined using 91 sera from unvaccinated children (ages 1 month to 10 years) presenting at the Rumuewhor Primary Health Centre in Emohua, Rivers State using standard ELISA. Relevant information was obtained using a Performa specially designed for the study. Of the 91 sera assayed, 79(86.8%) were positive for anti-MV-IgM antibodies and 12(13.2%) were negative. Forty-two (87.5%) males and 37(86.0%) females were positive for anti-MV-specific IgM antibodies. Seropositivity of anti-MV-IgM antibody was higher in age-group 0-8 months (100.0%), followed by age-groups 9 months-5 years (86.0%) while age-groups 6-10 years (72.2%) had the least seropositivity. The study showed lack of immunization (p<0.05) as the main factor of measles infection in the study area. Age of children and education of mothers were the main correlates and contributory to measles infection (p<0.05). Sex and history of previous exposure were not significantly associated (p>0.05). Seronegativity of anti-MV-IgM antibodies was low (13.2%). Group-specific seronegativity was also low (10.0–41.7%) though no significant connotation amid any of the socio-demographic characteristics and seronegativity of measles IgM was observed. This further queried if truly, measles vaccination is administered widely in Nigeria and thus able to prevent severe reinfection with the wild type. This study indicates that measles remains endemic in the area studied. The presence of measles in this community with unvaccinated children population can lead to measles outbreaks in the state. Ensuring timely vaccination, high vaccination coverage, early detection and quarantine, are important issues to limit measles spread.
Keywords: Antibodies, Immunization, Measles IgM, Seropositivity, Unvaccinated children
Cite this paper: Okonko Iheanyi Omezuruike, Jim-George Onari, Anti-Measles Virus (MV) IgM Antibodies in Unvaccinated Children Population in Emohua, Rivers State, Nigeria, Journal of Health Science, Vol. 7 No. 1, 2017, pp. 1-6. doi: 10.5923/j.health.20170701.01.
1. Introduction
- New eruptions of measles in low-income nations demonstrate that conservative approaches for assessing vaccination coverage do not sufficiently ascertain vulnerable children [1]. Measles remains one of the utmost significant etiologies of disease and deaths in emerging nations, subsequent in over a million deaths globally every year, in spite of the extensive obtainability of safe, active and effective vaccines [2]. The estimated mortality from measles rose from 122,000 in 2012 to 145,700 in 2013, and decreased to 114,900 in 2014 as development concerning the eradication of measles has delayed [3-5]. Ongoing outbreaks of measles have occurred mostly among unvaccinated communities [6]. In a recent outbreak of measles in Florida in 2013, none of the four children with confirmed measles were immunized against measles, and none had journeyed outside throughout the times when they likely had been open to the infection [5, 7, 8].In 2014, the United States has had an upsurge in measles activity from the time when the virus was eliminated in 2000 and this occurs mostly in unvaccinated persons [9].Unvaccinated population has become a major source of concern on recent measles outbreaks in developing and developed nations where high vaccination coverage has been reported. Most of the measles cases reported in 2013 in the United States have been in unvaccinated persons (200/288 [69.0%]) or persons with unknown vaccination status (58/288 [20.0%]); 30(10.0%) were in persons who were vaccinated [6]. Laboratory detection of measles is infrequently done in emerging nations and inclines to be contingent on scientific indications alone [10]. Serological analysis of Measles-specific IgM antibodies remains the standard examination for the early detection of measles, and is now wholly done using commercially available ELISA kits [11]. ELISA is the extensively suitable assay in most emerging nations like Nigeria. The ELISA method has been revealed to possess a high sensitivity [12].The outcome of detection of measles IgM in unvaccinated children poses a high risk to their health because measles can result to severe complications which may ultimately lead to mortality. Thus, the study sets to ascertain the seropositivity of anti-MV-specific IgM antibodies in unvaccinated children population of Emohua, Rivers State, Nigeria. It also seeks to evaluate the risk factors associated with the seropositivity for MV IgM antibodies among unvaccinated children and assess their susceptibility to the MV.
2. Methods
- Study areaThe study was carried out using unvaccinated children presenting at the Primary Health Centre, Rumuewhor in Emohua, Rivers State. Rumuewhor community is located in Emohua Local Government Area of Rivers State. Rumuewhor community comprises 4 villages (Rumuewhor, Rumuodugo I, Rumuodugo 11 and Eruewku). It has an estimated population of 18,722. It is a rural area where majority of the indigenes are farmers. There is a primary health centre owned and run by the state government which happens to be the only hospital in that community. This health centre carries out free medical care for adults and children which includes; immunization, maternal and child care and other health services. However, these free services are not well utilized by people in the community and as such there is a high level of unvaccinated children and adults. Study populationNinety-one (91) unvaccinated children attending the Rumuewhor Primary Health Centre in Emohua, Rivers State, Nigeria were enlisted after giving a written/verbal informed consent. Unwilling children were excluded. Socio-demographic characteristics of the children were obtained using structured questionnaire (Table 1). The sample size was determined as described by Macfarlane (13 and Naing et al. (14). Of the 91 children studied, 23(25.3%) was aged 0–8 months, 50 (54.9%) was aged 9 months to 5 years and 18 (19.8%) was children aged 6–10 years. Sample collection and plasma preparationA 3 mL blood samples were aseptically obtained from each unvaccinated children in a plain container with no anticoagulant. Samples were conveyed to the Medical Microbiology Unit, Department of Microbiology, University of Port Harcourt, Nigeria in a cold chain. The blood samples were centrifuged and the sera aspirated into Eppendorf tubes and kept at -20°C. Methods were in agreement with the ethical standards of the Nigerian National Code for Health Research Ethics and the Declaration of Helsinki (October 2008 revision).Serological testingThe samples were analyzed for anti-MV-specific IgM antibodies using immunoglobulin-M measles enzyme-linked immunosorbent assay kits (DIA.PRO Diagnostic Bioprobes, Milano, Italy). The serological testing and result interpretation was as stipulated by the kit’s manufacturer. Data Analysis Results were presented in proportions. We engaged Fisher's exact test and Chi-square test to evaluate variances amid groups at p < 0.05 significance. We used <1.0 as negative and ≥1.0 as positive in order to get valid analysis.
3. Results
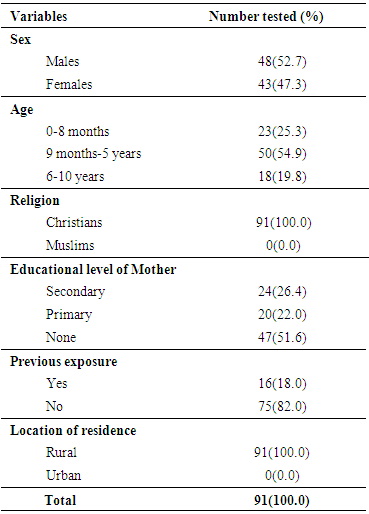
- Children characteristics The age-range of the 91 unvaccinated children population of Emohua was 0 month - 10 years. Age group 9 months – 5years had the highest frequency [50(54.9%)] in comparison of the other groups. This was followed by age group 0-8 months [23(25.3%)] while age groups 6-10 years were [18(19.8%)]. Ages were significantly dissimilar (P<0.05) [Table 1]. Twenty-four (26.4%) of the mothers had secondary level of education and 20(22.0%) had primary education while 47(51.6%) had no education level (Table 1). All (100.0%) of the participants were Christians and no Muslims (0.0%) and comparisons of the two religious groups were significantly different (P<0.05). Sixteen (18.0%) of the 91 children have had measles infection in the past while 75(82.0%) have not had any previous exposure to measles infection. All the children reside in the rural area (Table 1).
|
|
4. Discussion
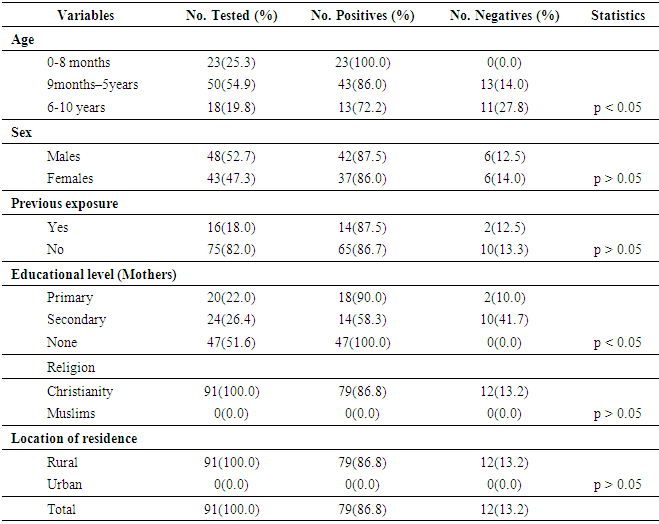
- In this study, MV-specific IgM antibody was evaluated in representative samples of the unvaccinated children population of Emohua, Rivers State, Nigeria using standard ELISA. Consequentially, seventy nine (86.8%) of the 91 the unvaccinated children tested had detectable IgM antibodies to MV. It showed that 79(86.8%) had detectable MV IgM antibody indicating recent or an ongoing measles virus infection. MV-specific IgM enzyme immunoassays were the suggested laboratory tests for the detection of severe measles infections [15]. This appeared to be adequate for measles control programs [15]. The 86.8% agrees with Olaitan et al. [2] who confirmed the presence of MV infection in children aged 0–8 months. It can be seen that MV remain endemic in the community studied and probably in Nigeria as a whole [2, 16]. This initial appearance of measles has been credited to declining maternal antibodies, particularly in places where immunity is from vaccination not natural infection [2].The 86.8% seropositivity reported in this study deviated from the values reported in Nigeria and elsewhere. It deviated from the 70.5% reported by Arista et al. [17], 20.9% by Oshitani et al. [10], 66.6% by Sekla et al. [18], 71.1% by Chukwu et al. [19], 30.2% by Bassey et al. [20], 71.7% by Rafiei et al. [21], 32.2% by Chechet et al. [16] and 21.2% by Olaitan et al. [2]. In agreement to this present finding, Sekla et al. [18] also detected specific-MV IgM antibodies in 89.2% of the convalescent sera from 28 vaccinated and 100.0% of the 17 unvaccinated clinical cases. The high seropositivity of 86.8% reported for anti-MV IgM antibody in this study could be due to the fact that the population studied had not received measles vaccine contrary to the recommended threshold (>90%) necessary to interrupt measles transmission [16, 22]. Current studies have identified several barriers to vaccinations in low income countries, which include lower parental education, lower maternal age, lower income, female gender of the child, traditional or Muslim religion, location of residence (urban versus rural), and larger family size [23, 24]. These were incorporated in this study. Though, an overall seropositivity of 86.8% was obtained, children aged 0–8 months had 100.0%, children aged 9 months–5 years 86.0% and 72.2% was reported in children aged 6-10 years. The seropositivity of anti-MV-specific IgM antibodies increase with age amongst children ages 0–8 months and declined with age amongst older children (ages 6–10 years), indicating substantial connotation (P<0.05) amid age and MV IgM seropositivity. The same pattern was also reported by Chukwu et al. [19], Bassey et al. [20] and Chechet et al. [16]. The 100.0% seropositivity of anti-MV IgM antibodies in children ages 0–8 months is in discordance with the 17% reported by Fauveau et al. [25], 8.0% by Adeboye et al. [26], 6.0% by Ahmadu et al. [27], 7.0% by Umeh and Ahaneku [28] and 6.5% by Olaitan et al. [2] in children < 9 months. The 86.0% seropositivity in children ages 9 months–5 years is in discordance with the 61.6% by Olaitan et al. [2] and 64.0% by Umeh and Ahaneku [28] in similar age-groups. Numerous authors have described the incidence of measles in infants under 9-months [29, 30]. Some have credited the incidence of measles amongst infants under 9-months to low affinity maternal measles antibody which accordingly upsurges the susceptibility of these infants to existing wild measles subtypes [31]. The 72.2% seropositivity amongst 6- 10 years children is in discordance with the 29.0% reported in similar age-groups in Abia State [28]. According to Orenstein et al. [32], the suitable age for measles immunization is ascertained by considering the danger of measles disease and complications at a specified age with vaccine effectiveness at that age. Thus, national vaccination schedules must define the ideal age for the initial dose by considering the danger of primary vaccine failure at younger ages contrary to the danger of MV infection before immunization [16, 22]. In most countries, the suggested age for routine immunization against measles is usually at 9 months of age [16, 32-35]. In Nigeria, this is presently put at nine months after birth [16, 33-35]. However, this age is unsuitable for several developing nations where the dangers of measles disease and complications are high in young pre-school children [32]. This is for the reason that reduced vaccine effectiveness, owing to unique host features such as nutritional status and sanitation or compromised vaccine or contact with circulating measles virus [16]. All these factors adversely affect seroconversion and could all play roles leading to measles infection in adulthood [16]. However, it is fascinating to know that all the unvaccinated children in this study have never received any vaccine at that age of their life. No sex (p>0.05) relationship with the seropositivity of anti-MV IgM in this study. This compared favourably with Rafiei et al. [21], Chukwu et al. [19] and Chechet et al. [16]. However, previous studies reported the contrary in other parts of Nigeria [2, 20] and other parts of the world [25, 36]. This finding may be connected with the general life style of male children [16]. Variation in strains of MV has accounted for the initial measles presentations, occurrence and reoccurrence of measles in vaccinated children [2, 37]. However, this study showed no important connotation (p>0.05) amid previous history of measles exposure and seropositivity of anti-MV-specific IgM antibodies. In this study, of the 16(18.0%) children with previous history of measles infection; 14(87.5%) were positive for anti-MV IgM and 65(86.7%) with no such history also tested positive. This agrees favourably with Sekla et al. [18] who indicated that a history of previous vaccination is not continuously related with the presence of specific antibodies or with immunity. In this study, educational level (p<0.05) was meaningfully related to the seropositivity of anti-MV IgM antibodies. This finding agrees with findings in Malawi [38] and Bolivia [36], where higher seropositivity was reported in children whose mothers had lower educational level compared to highly educated parents. However, it disagrees with Olaitan et al. [2] who indicated that measles infection remains endemic in the country; as such, all the general population is similarly exposed to the virus regardless of their educational standing.Seropositivity of anti-MV IgM antibody was only present among children who were Christian (86.8%). Communities and individuals in the United States decide on not to get vaccinated for various reasons, of which religious motives and philosophical reasons were chiefly cited [9]. Religion-based objections most frequently focused on animal-derived gelatins used in production of vaccines and aborted human fetus tissue used in the rubella constituent of the mumps, measles and rubella (MMR) combined vaccine products [9]. These objections amongst religious groups might also not be faith-based, somewhat in some cases were anxieties connected to lack of safety and effectiveness of the vaccination [9].In relation to location of residence, all the children used for this study lived in the rural areas. This agrees favourably with Umeh and Ahaneku [28] who reported that 75.0% of all measles cases happened in rural areas. Also, Fauveau et al. [25] reported that all cases of measles in their study occurred in rural Bangladesh. Seronegativity of IgM antibodies against MV in this study was low (13.2%). Group-specific negativity was low (10.0-41.7%) though no meaningful connection with any of the socio-demographic data and IgM seronegativity. Contrary to the 13.2% in this study, Chukwu et al. [19], Chechet et al. [16] and Nigatu et al. [39] reported 28.9%, 92.3% and 99.0% seronegativity respectively.
5. Conclusions
- This study reveals a seropositivity of 86.8% and 13.2% Seronegativity of anti-MV-specific IgM amongst unvaccinated children population of Emohua, Rivers State, Nigeria. It has further confirmed the presence of anti-MV-specific IgM in children ages 0 months-10 years with a seropositivity rates comparable to those elsewhere. It is therefore recommended that immunization be given to these children.
ACKNOWLEDGEMENTS
- The authors sincerely acknowledge the Management and staff of Rumuewhor Primary Health Centre, in Emohua, Rivers State, Nigeria for their cooperation and support. We also appreciate all the children and parents for their consent, cooperation and participation. We also thank Mrs. Mercy Elenwo for her assistance in collection of the samples.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML