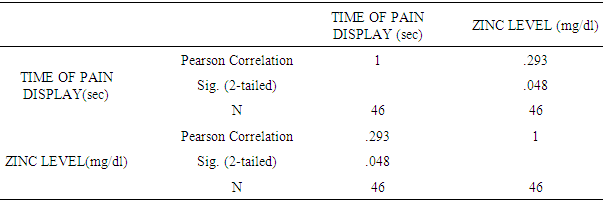
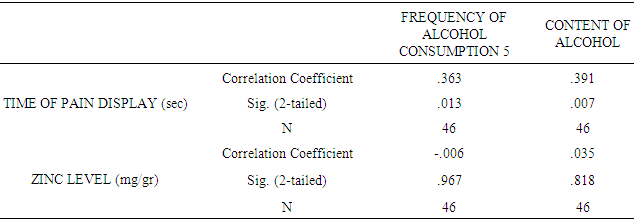
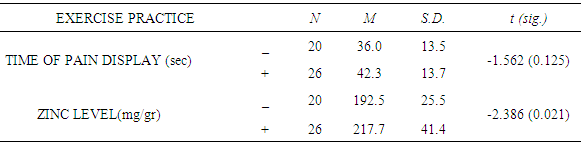
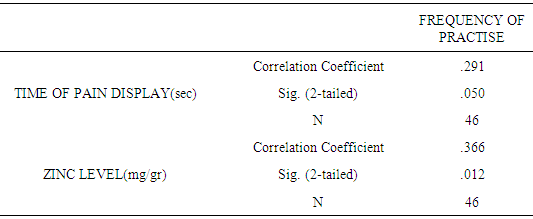
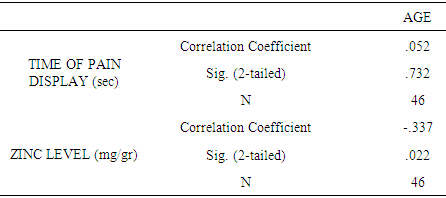
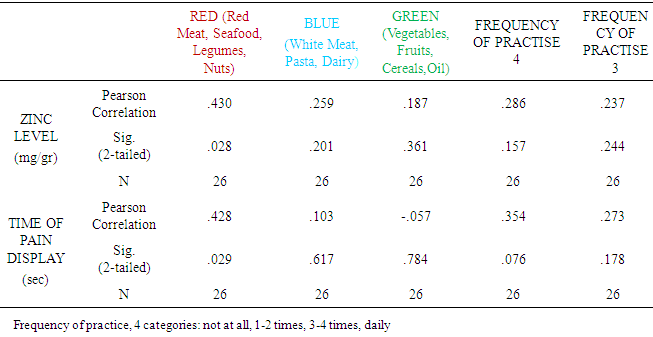
| [1] | King JC, Shames DM, Woodhouse LR Zinc homeostasis in humans. J. Nutr. 2000 May; 130(5S Suppl):1360S-6S. |
| [2] | Livingstone C. Zinc: physiology, deficiency, and parenteral nutrition. Nutr Clin Pract. 2015 Jun; 30(3):371-82. |
| [3] | Maywald M, Rink L. Zinc homeostasis and immunosenescence. J Trace Elem Med Biol. 2015 Jan; 29:24-30. |
| [4] | Bonaventura P, Benedetti G, Albarède F, Miossec P. Zinc and its role in immunity and inflammation. Autoimmun Rev. 2015 Apr; 14(4):277-85. |
| [5] | Grauert Antonia, Engel Dominique and Ruiz Arnaud J. Endogenous zinc depresses GABAergic transmission via T-type Ca2+ channels and broadens the time window for integration of glutamatergic inputs in dentate granule cells. J Physiol. 2014 Jan 1; 592(1): 67–86. |
| [6] | Chorin E, Vinograd O, et al. Upregulation of KCC2 activity by zinc-mediated neurotransmission via the mZnR/GPR39 receptor. J. Neurosci. 2011; 31(36):12916–12926. |
| [7] | Li C, Peoples RW, Weight FF. Inhibition of ATP-activated current by zinc in dorsal root ganglion neurones of bullfrog. J. Physiol. 1997 Dec 15; 505 (Pt 3):641-53. |
| [8] | Uchida Kunitoshi and Tominaga Makoto. Extracellular Zinc Ion Regulates Transient Receptor Potential Melastatin 5 (TRPM5) Channel Activation through Its Interaction with a Pore Loop Domain. Biol Chem. Sep 6, 2013; 288(36): 25950–25955. |
| [9] | Nozaki C, Vergnano AM, Filliol D, Ouagazzal AM, Le Goff A, Carvalho S, Reiss D, Gaveriaux-Ruff C, Neyton J, Paoletti P, Kieffer BL. Zinc alleviates pain through high-affinity binding to the NMDA receptor NR2A subunit. Nat Neurosci. 2011 Jul 3; 14(8): 1017-22. |
| [10] | Galan P, Viteri FE, Bertrais S, Czernichow S, Faure H, Arnaud J, Ruffieux D, Chenal S, Arnault N, Favier A, Roussel AM, Hercberg S. Serum concentrations of beta-carotene, vitamins C and E, zinc and selenium are influenced by sex, age, diet, smoking status, alcohol consumption and corpulence in a general French adult population. Eur J Clin Nutr. 2005 Oct; 59(10):1181-90. |
| [11] | Ozmen Habibe, Akarsu Saadet, Polat Fatih and Cukurovali Alaaddin. The Levels of Calcium and Magnesium, and of Selected Trace Elements, in Whole Blood and Scalp Hair of Children with Growth Retardation. Iran J. Pediatr. 2013 Apr; 23(2): 125–130. |
| [12] | Temiye EO, Duke ES, Owolabi MA, Renner JK. Relationship between Painful Crisis and Serum Zinc Level in Children with Sickle Cell Anaemia. Anemia. 2011; 2011:698586. |
| [13] | Ciubotariu Diana, Ghiciuc Cristina Mihaela and Lupușoru Cătălina Elena. Zinc involvement in opioid addiction and analgesia – should zinc supplementation be recommended for opioid-treated persons? Subst Abuse Treat Prev Policy. 2015; 10: 29. |
| [14] | Matsunami M, Kirishi S, Okui T, Kawabata A Chelating luminal zinc mimics hydrogen sulfide-evoked colonic pain in mice: possible involvement of T-type calcium channels. Neuroscience. 2011 May 5; 181: 257-64. |
| [15] | Tamba BI, Leon MM, Petreus T. Common trace elements alleviate pain in an experimental mouse model. J Neurosci Res. 2013 Apr; 91(4):554-61. |
| [16] | Anderson Charles T., Radford Robert J., Zastrow Melissa L., Zhang Daniel Y., Apfel Ulf-Peter, Lippard Stephen J. and Tzounopoulos Thanos. Modulation of extrasynaptic NMDA receptors by synaptic and tonic zinc. Proc Natl Acad Sci U S A. 2015 May 19; 112(20): 2705–2714. |
| [17] | Krystal JH, Staley J, Mason G, et al. Gamma-aminobutyric acid type A receptors and alcoholism: intoxication, dependence, vulnerability, and treatment. Archives of General Psychiatry. 2006; 63:957–968. |
| [18] | Hicklin TR, Wu PH, Radcliff PA, Freud RK, Goebel-Goody SM, Correa PR, Proctor WR, Lombroso PJ, Browning MD. Alcohol inhibition of the NMDA receptor function, long-term potentiation, and fear learning requires striatanal-enriched protein tyrosine phosphatase. Proc Natl Acad Sci USA. 2011 Apr 19; 108(16):6650-5. |
| [19] | Gutierrez Carlos A and Staehle Mary M. A control system analysis of the dynamic response of N-methyl-D-aspartate glutamate receptors to alcoholism and alcohol withdrawal. Theor Biol Med Model. 2015; 12:8. |
| [20] | Speich M, Pineau A, Ballereau F. Minerals, trace elements and related biological variables in athletes and during physical activity. Clin Chim Acta. 2001 Oct; 312(1-2):1-11. |
| [21] | Chu A, Petocz P, Samman S. Immediate Effects of Aerobic Exercise on Plasma/Serum Zinc Levels: A Meta-analysis. Med Sci Sports Exerc. 2015 Nov 4. |
| [22] | Savas S, Senel O, Okan I, Aksu ML. Effect of acute maximal aerobic exercise upon the trace element levels in blood. Neuro Endocrinol Lett. 2007 Oct; 28(5):675-80. |
| [23] | Somboonwong J, Traisaeng S, Saguanrungsirikul S, Moderate-intensity exercise training elevates serum and pancreatic zinc levels and pancreatic ZnT8 expression in streptozotocin-induced diabetic rats. Life Sci. 2015 Oct 15; 139:46-51. |
| [24] | Zhao J, Fan B, Wu Z, Xu M, Luo Y. Serum zinc is associated with plasma leptin and Cu-Zn SOD in elite male basketball athletes. J Trace Elem Med Biol. 2015 Apr; 30:49-53. |
| [25] | Scheef L, Jankowski J, Daamen M, Weyer G, Klingenberg M, Renner J, Mueckter S, Schürmann B, Musshoff F, Wagner M, Schild HH, Zimmer A, Boecker H. An fMRI study on the acute effects of exercise on pain processing in trained athletes. Pain. 2012 Aug; 153(8):1702-14. |
| [26] | Tesarz J, Gerhardt A, Schommer K, Treede RD, Eich W. Alterations in endogenous pain modulation in endurance athletes: an experimental study using quantitative sensory testing and the cold-pressor task. Pain. 2013 Jul; 154(7): 1022-9. |
| [27] | Afridi HI, Talpur FN, Kazi TG, Brabazon D. Estimation of toxic elements in the samples of different cigarettes and their effect on the essential elemental status in the biological samples of Irish smoker rheumatoid arthritis consumers. Environ Monit Assess. 2015 Apr; 187(4):157. |
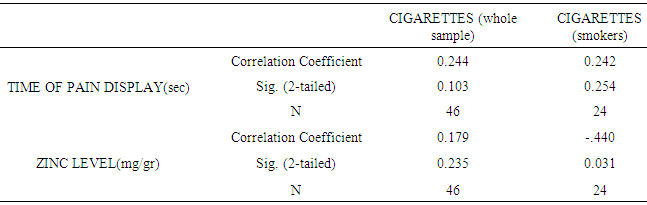
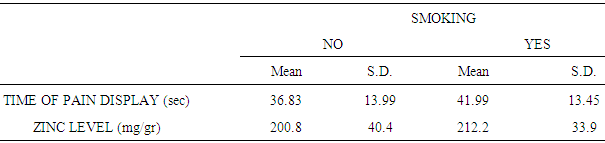
| [28] | Morales-Suárez-Varela María, Llopis-González Agustín, González-Albert Verónica, López-Izquierdo Raúl, González-Manzano Isabel, Cháves Javier, Huerta-Biosca Vicente and Martin-Escudero Juan C. Correlation of Zinc with Oxidative Stress Biomarkers. Int J Environ Res Public Health. 2015 Mar; 12(3): 3060–3076. |
| [29] | Afridi HI, Kazi TG, Kazi NG, Jamali MK, Arain MB, Sirajuddin, Baig J A, Kandhro GA, Wadhwa SK and Shah AQ. Evaluation of cadmium, lead, nickel and zinc status in biological samples of smokers and nonsmokers hypertensive patients. J. Hum Hypertens. 2010 Jan; 24(1): 34–43. |
| [30] | Liu RZ, Gao JC, Zhang HG, Wang RX, Zhang ZH, Liu XY. Seminal plasma zinc level may be associated with the effect of cigarette smoking on sperm parameters. J Int Med Res. 2010 May-Jun; 38(3):923-8. |
| [31] | Lönnerdal B. Dietary factors influencing zinc absorption. J Nutr. 2000 May; 130 (5S Suppl):1378S-83S. |
| [32] | Karen H. C. Lim, Lynn J. Riddell, Caryl A. Nowson, Alison O. Booth, and Ewa A. Szymlek-Gay. Iron and Zinc Nutrition in the Economically-Developed World: A Review. Nutrients. 2013 Aug; 5(8): 3184–3211. |
| [33] | Foster M, Samman S. Vegetarian diets across the lifecycle: impact on zinc intake and status. Adv Food Nutr Res. 2015; 74:93-131. |
| [34] | Haase Hajo and Rink Lothar. The immune system and the impact of zinc during aging. Immun Ageing. 2009; 6:9. |
| [35] | Mariani E, Cornacchiola V, Polidori MC, Mangialasche F, Malavolta M, Cecchetti R, Bastiani P, Baglioni M, Mocchegiani E, Mecocci P. Antioxidant enzyme activities in healthy old subjects: influence of age, gender and zinc status: results from the Zincage Project. Biogerontology. 2006 Oct-Dec; 7(5-6):391-8. |
| [36] | Hoogenboom Barbara J, Morris Jennifer, Morris Chad and Schaefer Katharine. Nutritional Knowledge and Eating Behaviors of Female, Collegiate Swimmers. N Am J Sports Phys Ther. 2009 Aug; 4(3): 139–148. |
| [37] | Dwyer Johanna, Eisenberg Alanna, Prelack Kathy, Song Won O, Sonneville Kendrin, and Ziegler Paula. Eating attitudes and food intakes of elite adolescent female figure skaters: a cross sectional study. J Int Soc Sports Nutr.2012; 9:53. |
| [38] | García-Rovés Pablo M, García-Zapico Pedro, Patterson Ángeles M. and Iglesias-Gutiérrez Eduardo. Nutrient Intake and Food Habits of Soccer Players: Analyzing the Correlates of Eating Practice. Nutrients. 2014 Jul; 6(7): 2697–2717. |


 Age group:
Age group: Birth place:Residence place during the last five (5) years:Education level:
Birth place:Residence place during the last five (5) years:Education level: Graduate from
Graduate from Occupation:Where did you work the last two years?b. Nutrition HabitsHow often do you consume, in a weekly basis, each of the food categories below:
Occupation:Where did you work the last two years?b. Nutrition HabitsHow often do you consume, in a weekly basis, each of the food categories below: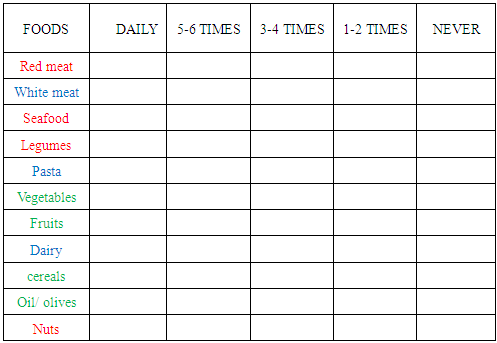 Food categories according to Zn content and bioavailability:
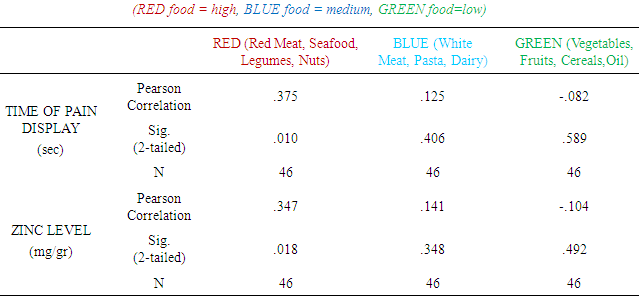
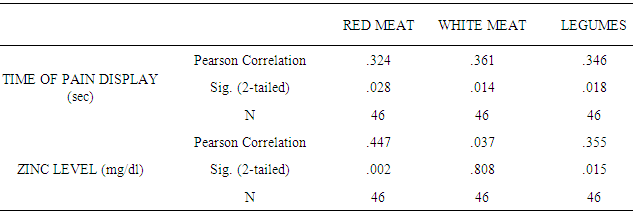
Food categories according to Zn content and bioavailability: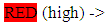 red meat (pork, calf), seafood (fishes, oysters, mussels), legumes, nuts
red meat (pork, calf), seafood (fishes, oysters, mussels), legumes, nuts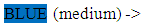 white meat, pasta, dairy products
white meat, pasta, dairy products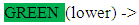 vegetables fruits, whole grain cereals, oil/olivesc. Life Style HabitsDo you smoke? :
vegetables fruits, whole grain cereals, oil/olivesc. Life Style HabitsDo you smoke? : If the answer is yes, how many cigarettes do you smoke? :
If the answer is yes, how many cigarettes do you smoke? : If the answer is yes, when did you start smoking? :
If the answer is yes, when did you start smoking? : ow often do you consume alcohol beverages? :
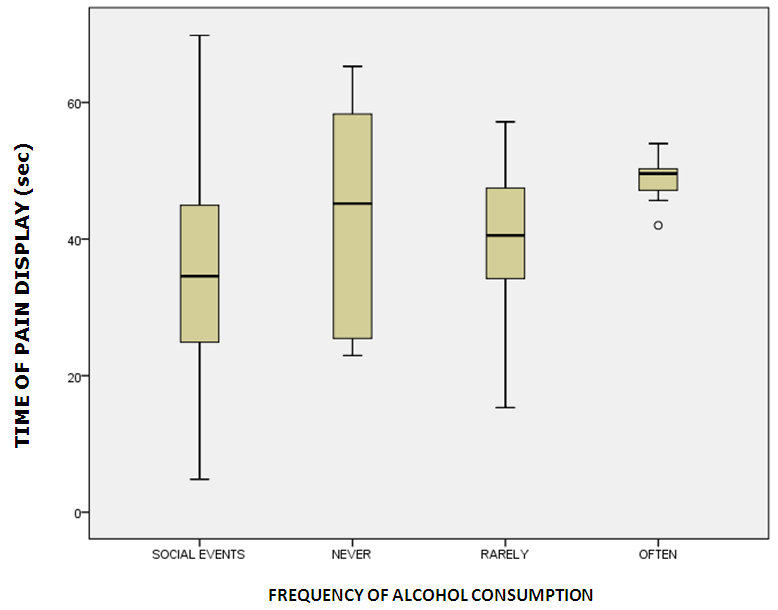
ow often do you consume alcohol beverages? : Only in social events and interactions:
Only in social events and interactions: 
 hich kind of beverages do you prefer? :
hich kind of beverages do you prefer? : Do you practice exercise / sport(Basketball, football, volleyball, water polo, gym, etc):
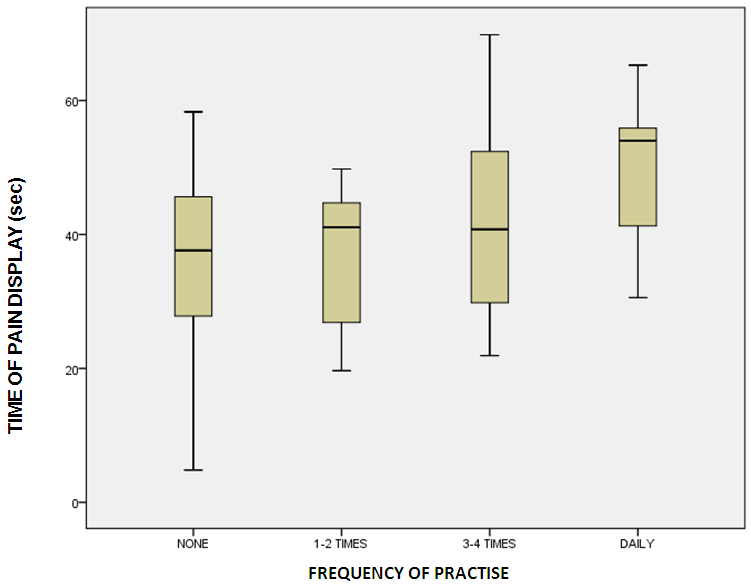
Do you practice exercise / sport(Basketball, football, volleyball, water polo, gym, etc): If you do, how often? :
If you do, how often? : d. Medical HistoryDo you have you any recent health problems? :
d. Medical HistoryDo you have you any recent health problems? : If the answer is yes, what kind of?Are you under any medical treatment? :
If the answer is yes, what kind of?Are you under any medical treatment? : If the answer is yes, what kind of?How long are you under medication? :
If the answer is yes, what kind of?How long are you under medication? : Do you take any Nutrition Supplements (NS)?:
Do you take any Nutrition Supplements (NS)?: If the answer is yes, what kind of?How long do you take NS? :
If the answer is yes, what kind of?How long do you take NS? : Are you allergic to? :medicaments:
Are you allergic to? :medicaments: food:
food: anything else:
anything else: Have you undergone any surgery? :
Have you undergone any surgery? : Do you suffer any chronic disease such as:
Do you suffer any chronic disease such as: Does any member of your family suffer a health problem? :
Does any member of your family suffer a health problem? :
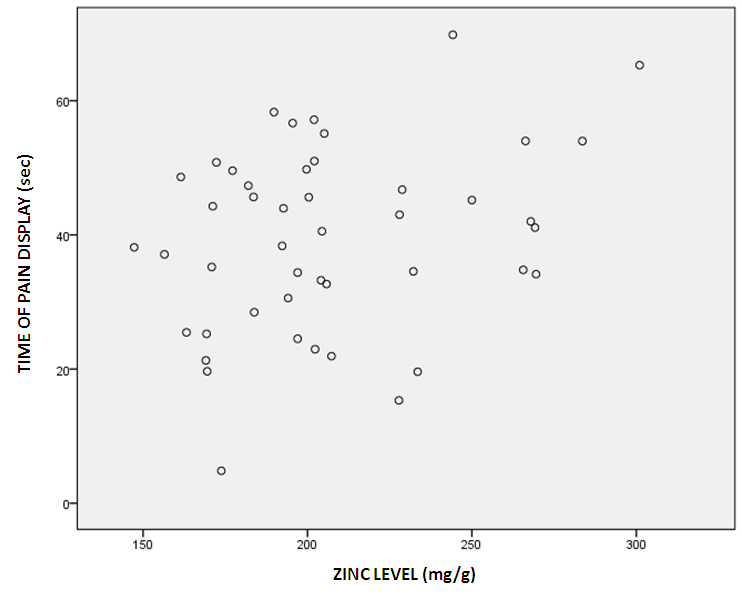
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML