-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Health Science
p-ISSN: 2166-5966 e-ISSN: 2166-5990
2016; 6(3): 37-42
doi:10.5923/j.health.20160603.01

Clinical and Echocardiographic Profile of Patients with Atrial Fibrillation in Nigeria
Ebenezer Adekunle Ajayi 1, Victor Oladeji Adeyeye 2, Adekunle Olatayo Adeoti 1
1Department of Medicine, Ekiti State University Teaching Hospital, Ado Ekiti, Nigeria
2Department of Medicine, Obafemi Awolowo University Teaching Hospital Complex, Ile Ife, Nigeria
Correspondence to: Ebenezer Adekunle Ajayi , Department of Medicine, Ekiti State University Teaching Hospital, Ado Ekiti, Nigeria.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia encountered in clinical practice requiring treatment. There is a paucity of data on AF in Nigeria; hence, this study was undertaken with the aim to characterize the clinical and echocardiographic profile of patients with AF in our hospital. Materials and Methods: This observational hospital-based study was carried out on 55 patients with electrocardiographically diagnosed AF seen at a sub-urban tertiary hospital in Nigeria from January 2012 to December 2015. All patients who were reviewed clinically also had transthoracic 2-D echocardiography done. Results: There were 29 (52.7%) males. Patients mean age was 67.40 ±13.83 years with a stepwise increase in the frequency of AF as age increases. Hypertension (87.3%) was the commonest associated co-morbidity. Mean CHADS2 score was 2.38 ± 1.35 and 65.5% had CHADS2 score ≥ 2. Dyspnea was the commonest presenting symptom in 67.3% of the patients. Echocardiographic left atrial enlargement (LAE) and left ventricular hypertrophy (LVH) was seen in 60% and 89.1% of the patients respectively. The commonest echocardiographic diagnosis was hypertensive heart disease (65.5%) followed distantly by dilated cardiomyopathy in 16.4%. Conclusions: Increasing age and hypertension are associated with occurrence of AF. As majority of our patients with AF fall into high risk of stroke category, early institution of appropriate treatment modalities will help reduce the morbidity and mortality associated with stroke. Presence of LVH or LAE in patients with hypertensive heart disease requires aggressive management to delay the development of AF.
Keywords: Atrial fibrillation, Clinical presentation, Etiological profile, Echocardiography
Cite this paper: Ebenezer Adekunle Ajayi , Victor Oladeji Adeyeye , Adekunle Olatayo Adeoti , Clinical and Echocardiographic Profile of Patients with Atrial Fibrillation in Nigeria, Journal of Health Science, Vol. 6 No. 3, 2016, pp. 37-42. doi: 10.5923/j.health.20160603.01.
1. Introduction
- Atrial fibrillation (AF), a phenomenon characterized by disorganized atrial activation and un-coordinated contraction, is the most common sustained cardiac arrhythmia encountered in clinical practice requiring treatment. It is the most common sustained cardiac arrhythmia in the developed world; and its prevalence in Africa is expected to grow as risk factors for AF, includ-ing an aging population, increase in this region [1, 2].In the developed world, the prevalence of AF averages 1% and increases with age, such that 10% of the population over the age of 80 has AF, and approximately 70% of cases of AF are in patients between 65 and 85 years of age [3]. Data on the prevalence and incidence of AF in Africa are rather sparse and not as robust as in developed world. Furthermore, available data suggested that the prevalence of AF in Africa currently is lower than in the western world [4]. In Africa, hospital-based studies put the prevalence of AF at approximate 5% [5-9]. For instance, in South Africa, 4.6% of cardiology patients had AF, indicating an estimated prevalence of 5.6 cases per 100,000 population per year [5]. Similar studies in Ivory Coast [6], Senegal [7], Nigeria [8] and Kenya [9] put the prevalence at 5.5%, 5.4%, 3.5% and 0.7%, respectively. However, a community- based study in a rural area in Ghana reported a prevalence of 0.3% among 924 individuals aged 50 years and older [10]. It is expected that there will be about 150% increase in the number of patients with AF by 2050 globally, possibly driven by the increasing aging of the population [11].Established risk factors for AF include known cardiovascular risk factors, such as systemic hypertension, diabetes mellitus, obesity, and cigarettes smoking, metabolic syndrome [12-14]. Others are echocardiographic evidence of structural heart disease, such as left atrial (LA) enlargement and left ventricular hypertrophy (LVH) [15].There is a paucity of data on AF in the study area. Hence, this study was undertaken with the aim to characterize the clinical and echocardiographic profile of patients with AF in our hospital.
2. Material and Methods
- This was an observational hospital-based study on clinical and echocardiography profile of patients with atrial fibrillation carried out at a sub-urban tertiary hospital in Southwest Nigeria from January 2012 to December 2015. The study protocol was approved by the Research and Ethics Committee of our hospital. All the participants gave written informed consent. All adult patients seen within the period with an electrocardiographic diagnosis of AF were reviewed clinically to determine the presenting symptoms, morbidities and risk factors for AF. They further had laboratory investigations including serum lipid profiles and transthoracic 2-D echocardiography. Subjects were considered diabetic if they had fasting plasma glucose > 126mg/dl (7.0mmol/L) [16] or if they used hypoglycemic medications and were considered hypertensive if they had a resting SBP >140mmHg and/or DBP > 90mmHg on two occasions measured after at least 3 minutes of rest with a mercury sphygmomanometer or if they were on antihypertensive therapy [17].The transthoracic two-dimensional (2D) and 2D derived M-mode echocardiography was performed according to standard procedure [18], while in the left lateral decubitus position using the SonoScape 1000 Ultrasound Imaging System with 2-4MHz transducer. Left ventricular end-diastolic measurements were taken during at least three cardiac cycles according to American Society of Echocardiography convention [19]. These included the left ventricular internal diameter (LVIDD), posterior wall thickness (PWT) and interventricular septal thickness (IVST). Left Ventricular (LV) mass was estimated from the Devereux’s formula [20] = {0.80 (1.04[(LVIDD + PWT + IVST)3 - LVIDD3]) + 0.6}g and normalized to height2.7 (ht2.7) to give left ventricular mass index (LVMI).Upper normal limits for LV mass index were >48 g/ m 2.7 in men and >44 g/ m2.7 in women [21] to define LVH. Relative wall thickness (RWT) was defined as (2 × PWT)/ LVIDD [21]. A partition value of 0.42 for relative wall thickness was used for both men and women [19]. Patients with increased LV mass index and increased RWT were considered to have concentric hypertrophy, and those with increased LV mass index and normal RWT were considered to have eccentric hypertrophy. Those with normal LV mass index and increased or normal RWT were considered to have concentric remodeling or normal geometry, respectively [21]. Left atrial anteroposterior linear dimension (LAD) obtained from the parasternal long axis view was taken from the trailing edge of the posterior aortic wall to the leading edge of the posterior left atrial wall at the end-ventricular systole when the LA chamber was at its greatest dimension [21].LA changes were defined as LA diameter >4.0cm and >3.8cm in males and females, respectively. Gender- specific thresholds 4.1-4.6/3.9-4.2cm, 4.7-5.2/4.3-4.6cm and ≥5.2/4.7cm were used to grade severity of abnormality as mild, moderate and severe in men/women, respectively [21]. LA diameter in relation to aortic root diameter (LAD/AOD) to define disproportionate LA enlargement as LAD/AOD >1.4 [22].The cut – off for abnormal values for serum total cholesterol, serum low-density lipoprotein and serum triglycerides were ≥5.18 mmol/L, ≥2.59 mmol/L and ≥ 1.7 mmol/L, respectively [21]. For serum high-Density lipoprotein, gender- specific abnormal values were <1.03 mmol/L (men) and < 1.29 mmol/L (women) [23].All statistical analyses were performed with computer program IBM SPSS 20 (IBM Corporation, 2011). Results were expressed as mean ± Standard Deviation (SD) and frequency expressed as a percentage.
3. Results
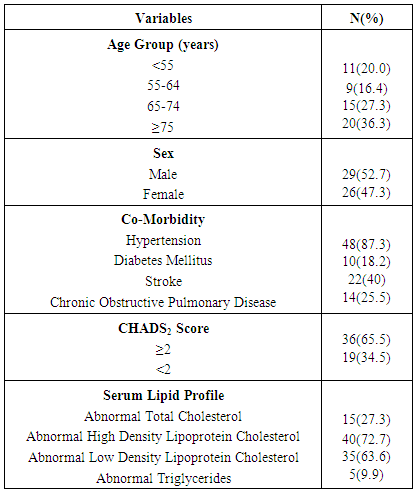
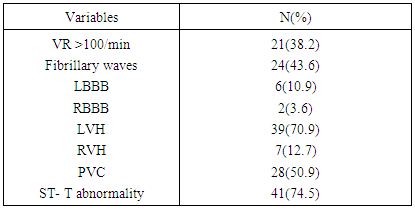
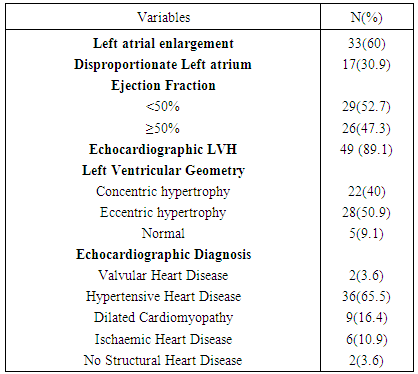
- Out of total ECG tracings of 1462 patients, 55 patients (3.76%) were seen with electrocardiographic diagnosis of AF comprising 29 (52.7%) males and 26 (47.3%) females. Their mean age was 67.40 ±13.83 years with age ranging from 34 - 89 years. As shown in Table 1, 80% of the patients aged 55years and above and 63.6% aged 65 years and above. There was a stepwise increase in the frequency of AF as the age increases. Body mass index (BMI) was ≥ 25kgm-2 in 41(74.5%) of the patients, out of which 3(5.5%) had BMI > 30kgm-2. Hypertension (87.3%) was the commonest associated co-morbidity. Others were: stroke (40%), diabetes mellitus (18.2%), and chronic obstructive pulmonary disease (25.5%). The mean CHADS2 score was 2.38± 1.35. A large proportion (65.5%) had CHADS2 score ≥ 2. Most of the patients had abnormal serum cholesterol, predominantly abnormal high density lipoprotein (72.7%) and low density lipoprotein (63.6%).The commonest presenting symptom was dyspnea, seen in 67.3% of the patients. As shown in Table 2, 10.9% had no symptom at the time of presentation.Table 3 shows that 38.2% presented with ventricular rate > 100/minute on ECG. Fibrillary waves were seen in 43.6% and 50.9% had premature ventricular complexes in addition. ECG-LVH and ECG-RVH were seen in 70.9% and 12.7% of the patients. Left bundle branch block and right bundle branch block were present in 10.9% and 3.6%, respectively. ST depression and T wave inversion were s seen in 74.5%.As seen in Table 4 and Figure 1, echocardiographic left atrial enlargement was seen in 60% of the patients with varying degree of severity comprising mildly severe (21.8%), moderately severe (20%) and severe (18.2%). Other forms of left atrial abnormalities seen in the patients are also shown in Table 4. Echocardiographic LVH was seen in 89.1% of the patients. Eccentric left ventricular geometry was the commonest phenotype seen in 50.9% while concentric left ventricular geometry was seen in 40%. The remaining 5(9.1%) had normal left ventricular geometry. The commonest echocardiographic diagnosis was hypertensive heart disease (65.5%) followed distantly by dilated cardiomyopathy seen in 16.4% (Table 4).
|
|
|
|
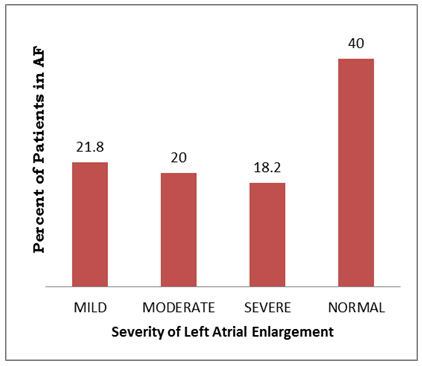 | Figure 1. Severity of left atrial enlargement |
4. Discussion
- The slight preponderance of AF in males compared with females seen in our study was also reported in another study by Anisiuba et al [8] in the Eastern part of Nigeria. A previous report on global epidemiology of AF had also indicated preponderance of male in patients with AF in both developed and developing countries [2]. Our study also showed that there was an increasing occurrence of AF with increasing age of the patients which agrees with previous studies [1, 3]. The link of AF with established cardiovascular risk factors whose occurrences also increase with age is a possible explanation. The mean age of our patients is in agreement with the mean age reported in AFIB Cameroon study [24]. However, the mean age of our study is higher than the mean age in different regions of the world according to reports from RE-LY AF study registry [25]. This is possibly due to the predominance of non-valvular etiology of AF in our patients compared with those other studies where rheumatic valvular heart disease was the commonest etiology. Furthermore, in areas where rheumatic heart disease is very prevalent, valvular AF predominates and patients are usually younger than those with non-valvular AF [25].Most of our patients had structural heart disease with hypertensive heart disease as the commonest etiology, followed distantly by dilated cardiomyopathy. The predominance of hypertensive heart disease as the commonest echocardiographic diagnosis in AF patients was documented in the AFIB Cameroon study [24] similar to our study. Rheumatic valvular heart disease was also common in the AFIB Cameroon study in contrast with our study where it had a very low frequency. A low prevalence of rheumatic valvular heart disease but high prevalence of hypertension-related cardiovascular admissions has been reported in our hospital [26]. We are of the opinion that the high prevalence of hypertension in Nigeria and the aged population of our patients might also explain this finding. Chronic exposure of the cardiovascular system to hypertension leads to left ventricular pressure overload with increased left atrial pressure, left atrial enlargement and left ventricular hypertrophy as sequelae. In addition, the chronic stimulation of the Renin-Angiotensin-Aldosterone axis leads to myocardial and atrial fibrosis. These pathophysiological adaptations are known to predispose to AF. In contrast with the large proportion of idiopathic cases of AF described in western populations, AF in African populations are usually accompanied by underlying cardiac disorders in proportions up to 90%, quite often, hypertensive heart disease and rheumatic valvular heart disease [27, 28].Cardiac structural adaptations were obvious in the majority of our patients. For instance, most of our patients had both ECG-LVH (70.9%) and Echocardiographic LVH (89.1%) with the common phenotypes being eccentric (50.9%) and concentric (40%) left ventricular geometry, respectively. Echocardiographic left atrial enlargement was present in 60% in this study in contrast to 95.7% in the AFIB Cameroon study despite comparable mean age with our study. However, Kumar et al [29], in India, reported left atrial enlargement in 68% of their AF patients. About half of our patients had worse than mild left atrial enlargement. Sanflippo et al [30] opined that atrial size is increased in time with atrial fibrillation, even in the absence of other causes of atrial enlargement. Only 38.2% of our patients had ECG feature of AF with a fast ventricular response. This is in contrast to two different studies in India by Kumar et al [29] and Kumar et al [31], where fast or rapid ventricular response was reported to be 69% and 85%, respectively. The ventricular response and thus ventricular rate in AF is dependent on several factors including vagal tone, other pacemaker foci, atrioventricular node function, refractory period, and medications. The reason for the low rate of fast ventricular response in our patients is not clearly known but it suffices to note that 50.9% of our patients also had premature ventricular complexes in addition to AF, suggestive of the presence of other pacemaker foci. We found 43.6% of our patients with fibrillary waves on ECG in contrast to 71-72% [29, 31] in some Indian populations. Obesity and the metabolic syndrome have been linked to increased risk of AF. In the prospective Framingham Heart study, obesity was associated with a 4% to 5% increased risk of AF over a mean follow-up of 14 years, independently of hypertension, diabetes, and myocardial infarction [32]. The Women’s Health study reported a linear relationship between body mass index (BMI) and AF, with a 5% increase in risk of AF for a 1-unit increase in BMI [33]. Apart from high prevalence of hypertension in our study population, other features of metabolic syndrome found were overweight/obesity (74.5%) and abnormally low HDL-cholesterol (72.7%). The relationship between these factors and AF may also be mediated by an increase in left atrial diameter, a risk factor that can be at least partially attenuated by weight loss [34]. One of the most commonly used and well-validated methods is the CHADS2 score which stratifies patients by the history of Congestive heart failure, Hypertension, Age >75 years, diabetes mellitus (one point each), and Stroke or transient ischemic attack (two points) [35]. Patients with a CHADS2 score of zero are considered to be at low risk of stroke; those with a score of one are considered to be at moderate risk while those with a score ≥2 are considered to be at high risk. In our study, 65.5% had a CHADS2 score ≥2 compared to 64.5% seen in AFIB Cameroon study and 65% in Kenya [9]. In a European cohort, 20% of patients had a CHADS2 score of zero, 62% had a score of one or two, and 18% had a score ≥3 [36]. The mean CHADS2 score in our patients was 2.38 ± 1.35 which is higher than 1.9±0.1 reported in AFIB Cameroon study and 1.5±0.9.2 in a hospital-based study in South Africa. This high mean CHADS2 score in our patients study population might have accounted for the about one- fifth of our patients as against one- tenth in a report by Kumar [29] that presented with current or past history of stroke. Thus, similar to other regions of the world, most of our patients with AF in this study are at moderate-to-high risk of stroke. It has been reported that patients with AF have a five-to seven-fold greater risk of stroke than the general population [37] and strokes secondary to AF have a worse prognosis than in patients without AF [38].In terms of symptomatology, 67.3% of our patients, similar to 74% by Kumar et al [29], 72.1% in AFIB Cameroon study [24], 62% in the study by Tischler et al [39] and 78% by Flaker et al [40] presented with history of dyspnea. Palpitation was the presenting complaint in 18.2% of our patients in contrast to 57% reported by Kumar et al [29], 54.1% reported by Levy et al [41] and 46.5% in AFIB Cameroun study [24]. This low percentage of palpitation as a presenting complaint in our patients might have mirrored the fact that most of the patients did not have a fast or rapid ventricular response to their AF as earlier noted. Our study is not without limitations as it is hospital-based with small patients’ population and cannot be generalized to the entire population. Patients with paroxysmal AF might have been missed by the instrument of this study.In conclusion, male sex, increasing age and hypertension are associated with occurrence of AF in our study. As over 65% of our patients with AF fall into high risk of stroke category, early characterization and institution of appropriate treatment modalities of such patients will help reduce the morbidity and mortality associated with stroke. Another key lesson to learn from this study is that the presence of LVH or left atrial enlargement in patients with hypertensive heart disease requires aggressive management to delay the development of AF and its attendant increased morbidity and mortality.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML