-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Health Science
p-ISSN: 2166-5966 e-ISSN: 2166-5990
2016; 6(1): 12-16
doi:10.5923/j.health.20160601.03

Alcohol Consumption among Pregnant Women Detected by Carbohydrate Deficient Transferrin
Andrea Guala1, Enrico Finale2, Alberto Cerutti3, Nino Cappuccia3, Roberta Amadori2, Giovanna Patrucco4, Chiara Crosa Lenz5, Cesare Danesino6
1Department of Pediatrics, Castelli Hospital, Verbania, Italy
2Department of Obstetrics and Gynaecology, Castelli Hospital, Verbania, Italy
3Clinical Laboratory, Castelli Hospital, Verbania, Italy
4Clinical Laboratory, S.Andrea Hospital, Vercelli, Italy
5Center on Alcoholism, Substance Abuse, and Addictions, San Biagio Hospital, Domodossola, Italy
6Institute of Medical Genetics, University of Pavia, IRCCS S.Matteo Hospital, Pavia, Italy
Correspondence to: Enrico Finale, Department of Obstetrics and Gynaecology, Castelli Hospital, Verbania, Italy.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The aim of this study was to detect the pattern of alcohol consumption among a cohort of pregnant women living in a north-western Region in Italy (Piedmont). We performed a cross-sectional study conducted between 2010 and 2011. A total of 603 women were recruited as a consecutive cohort. Using a conventional cut-off concentration of % CDT (carbohydrate deficient transferrin) >1.7%, corresponding to 2 SD above the mean or a concentration above the 97.5th percentile for non-pregnant controls, 6.4% of the samples (28/603) were classified as being ‘positive’ for this biomarker at recruitment. Only 7 patients (1.16%) had a %CDT concentration above 2%, corresponding to more than 3 SD above the mean for non-pregnant controls. They were therefore identified to be positive for alcohol consumption. In our study we found that 1.16% of pregnants had a high % CDT concentration.
Keywords: Alcohol consumption in pregnancy, Fetal alcohol syndrome, Fetal alcohol spectrum disorders, Carbohydrate deficient transferrin
Cite this paper: Andrea Guala, Enrico Finale, Alberto Cerutti, Nino Cappuccia, Roberta Amadori, Giovanna Patrucco, Chiara Crosa Lenz, Cesare Danesino, Alcohol Consumption among Pregnant Women Detected by Carbohydrate Deficient Transferrin, Journal of Health Science, Vol. 6 No. 1, 2016, pp. 12-16. doi: 10.5923/j.health.20160601.03.
Article Outline
1. Introduction
- Fetal alcohol syndrome (FAS) or fetal alcohol spectrum disorders (FASD) are a pattern of mental and physical defects that can develop in a fetus in association with high levels of alcohol consumption during pregnancy [1]. Preventing and reducing the harmful use of alcohol is a public health priority; alcohol consumption and problems related to alcohol vary widely around the world but the burden of diseases and deaths remains significant in most countries [2]. Estimates of the prevalence of FAS/FASD primarily depend on the diagnostic criteria and guidelines currently available [3]. The problem lies therein: the aforementioned criteria are ill-defined. In the absence of a reliable biochemical marker of effects to confirm maternal drinking during pregnancy, the importance and dependence on diagnostic guidelines for FAS/FASD is understated [4].Self-reported maternal alcohol abuse during pregnancy is not reliable and ethanol consumption is rarely admitted [5]. Commonly performed laboratory tests for alcohol consumption such as carbohydrate deficient transferrin (CDT), liver enzymes such as gamma-glutamyl-transferase (GGT), and mean corpuscular volume (MCV) are indirect alcohol markers. They are difficult to interpret and insufficiently reliable during pregnancy [6]. Other parameters for alcohol consumption exist as direct metabolites of ethanol degradation. They can be found in blood, urine, hair, and meconium. Fatty acid ethyl esters (FAEE) in meconium have been investigated and established in several studies as biomarkers of fetal ethanol exposure during the last 3 months of pregnancy [7]. Additionally, the determination of ethylglucuronide (EtG) not only from the mothers’ hair or urine but also from the children’s meconium has been associated with the mother’s drinking behavior during pregnancy [8, 9].Carbohydrate deficient transferrin (CDT) is a specific serum biomarker of heavy alcohol consumption, defined as ≥ 350–420 g alcohol/week. CDT refers to a temporary alteration in the glycosylation pattern of transferrin resulting in an increase in the relative amounts of disialo- and asialo-transferrin (and a decrease in tetrasialotransferrin) that occurs as a result of sustained heavy alcohol consumption (thresholds range from 50 to 80 g of alcohol/day for at least 2 weeks) [10]. Altered transferrin glycosylation patterns return to baseline levels within 2 to 5 weeks following complete abstinence from alcohol [11]. Using the standardized reference measurement technique with high performance liquid chromatography (HPLC) and quantification of disialotransferrin as a percentage of total transferrin (%CDT), a value of > 1.7 is considered to be specific for sustained heavy alcohol consumption [12]. Some prior studies, which used older techniques to measure CDT, indicated that serum CDT concentrations are higher in non-pregnant women than in men [13]; however, studies based on HPLC analysis found no gender differences [14]. Furthermore, recent reports suggested that the disialo-isoform increases with advanced gestational age during, which, based on the current cut offs of 1.7%, could lead to false positive results [15, 16]. A prospective study examined %CDT changes in 24 pregnant women by analyzing an average of seven repeated samples per person. Their results indicated that %CDT levels increased from 1.07 ± 0.17% during the 9th to the 16th weeks of gestation to 1.61 ± 0.23% in samples obtained within 1 week prior to delivery (~50% increase) and that the levels began to increase in the second trimester. Moreover, that study demonstrated that all CDT glycoform levels returned to the baseline concentrations within 8–15 weeks postpartum. However, no other biomarkers were evaluated in that study to confirm false-positive results. These results suggest that a conventional cutoff of 1.7% CDT might be too low for pregnant women and would generate false-positive results, especially if evaluated near or at term. We therefore chose a %CDT of ≥ 2.0% to be considered as a threshold concentration indicative of alcohol exposure in pregnant women [17].The majority of studies related to FAS/FASD are from the U.S., and most have utilized clinic or record-based systems without active recruitment [18]. Such methods under-report prevalence and describe only the most severe cases [19]. A first wave of research in this area of Italy revealed many alcohol-linked disabilities and a FAS/FASD prevalence of 3.7 to 7.4 per 1,000. Overall FAS/FASD was estimated as 35 per 1,000 (3.5%) [20, 21]. The aim of this study was to detect the pattern of alcohol consumption among a cohort of pregnant women living in a north-western Region in Italy (Piedmont).
2. Materials and Methods
- This was a cross-sectional study conducted between 2010 and 2011. A total of 603 women were recruited as a consecutive cohort. The participating women had to be aged ≥ 18 years with gestational age between 36 and 41 weeks, delivering at the Department of Obstetrics and Gynecology of “Castelli Hospital” in Verbania and “S. Biagio Hospital” in Domodossola. There was no preselection of the cases with respect to suspected alcohol abuse of the mother or any other parameter. Data on maternal and newborn perinatal outcomes (e.g. gestational age at delivery, complications during pregnancy) were collected from the medical records. Data and blood samples were anonymously treated therefore it was impossible to identify the participants or make connections with age, ethnicity and obstetric parameters. Fisher's exact test, Student's t-test, and multiple logistic regression analysis were performed.
2.1. CDT Analysis
- Upon admission to the hospital for delivery, serum was collected and stored at -20°C. CDT analysis were performed on a CZE (SEBIA, Lisses, Francia) System. The currently accepted laboratory reference value indicative of heavy drinking in pregnancy was % CDT ≥ 2.The choice not to cross the indirect markers of alcohol consumption [liver enzymes, gamma-glutamyl-transferase (GGT) and mean corpuscular volume (MCV)] comes from the inability to connect the values of CDT with the values of the other markers as the sample was anonymous.This study was approved by the Ethics Committee of Novara and all patients gave informed consent.
3. Results
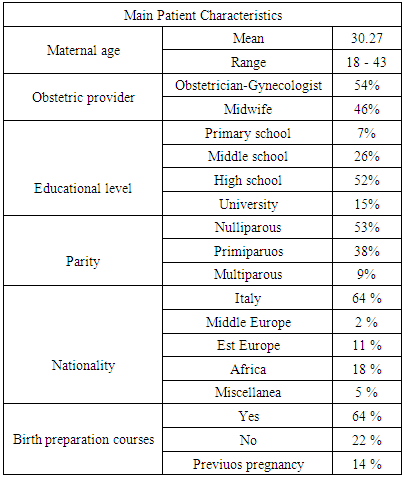
3.1. Main Patient Characteristics
- The mean maternal age at recruitment was 30,27 ± 2,12 years (range 18-43 years) (Tab 1).
|
3.2. Main Measurement Characteristics
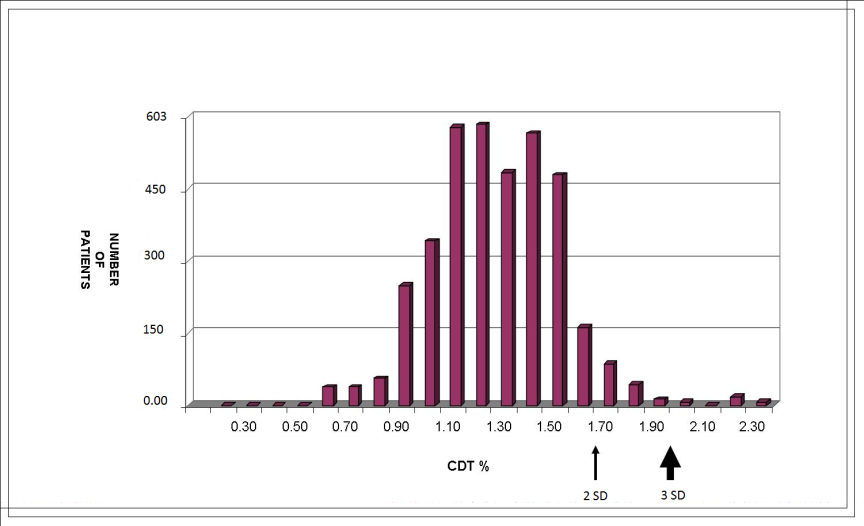
- Using a conventional cut-off concentration of %CDT >1.7%, corresponding to 2 SD above the mean or a concentration above the 97.5th percentile for non-pregnant controls, 6.4% of the samples (28/603) were classified as being ‘positive’ for this biomarker at recruitment. Only 7 patients (1.16%) had a %CDT concentration above 2%, corresponding to more than 3 SD above the mean for non-pregnant controls. They were therefore identified to be positive for alcohol consumption. Distribution of the corresponding CTD% in this population is shown in Fig. 1.
4. Discussion
- In the past 20 years, Italian population lifestyle has radically changes, especially for women, who have adopted behavioural models traditionally associated with men. One of the most important changes has been the increasing number of women who smoke, and more recently, those who drink. These habits that can be considered as a reflection of the changing role of women in society and they obviously have an important impact on the health status of female population [22].The problem with drinking alcohol during pregnancy is that there is no amount that has been proven to be safe. Researchers do not know enough about the potential effects of drinking alcohol at particular times during the pregnancy. Because there are so many uncertainties, the American College of Obstetricians and Gynecologists and the American Academy of Pediatrics advise pregnant women not to drink alcohol at all. Use of alcohol during pregnancy can lead to FAS/FASD and other harms such as spontaneous abortion, stillbirth, low birthweight, prematurity and birth defects. Use of alcohol and other drugs can also severely impair an individual’s functioning as a parent, spouse or partner, and trigger gender-based and domestic violence, thus significantly affecting the physical, mental and emotional development of children [23].Screening women of child-bearing age and pregnant women and recording their alcohol consumption is a practical process which could help to identify and evaluate women at risk and to identify potentially exposed infants. After validation, alcohol screening could be implemented systematically in prenatal care as well as postnatal identification of women being at high risk of child neglect [24].Maternal alcohol consumption must be identified in order to provide effective counselling support and treatment for women who are drinking at levels that might compromise their health or the health of their babies. Asking questions about alcohol use during pregnancy is necessary for gathering accurate and reliable information that will initiate an appropriate intervention program, as well as for early diagnosis of babies affected by prenatal alcohol exposure [25]. Traditional population monitoring is primarily dependent on maternal self-reporting, which is highly ineffective in identifying prenatal alcohol consumption because of the associated stigma [26]. Alcohol and uncontrolled substance abuse in pregnancy is under-recognized by traditional means of public health monitoring. This contributes to the substantial lack of dedicated programs that provide supportive intervention for women and neonates at risk and perpetuates the misperception among general practitioners, paediatricians and obstetricians that prenatal alcohol exposure does not occur within their clinical population [27].The prevalence of alcohol use in European pregnant women ranged from 12 to 63% and binge drinking ranged from 1 to 7% [28]. In our study we found that 1.16% of pregnants had a high %CDT concentration, less than expected. But it is an important percentage, mainly because in our study cohort most of the women (67%) declared more than 12 years of education and the majority (64%) attended birth preparation classes. Previous literature demonstrate that continued drinking in pregnancy is associated with older maternal age and higher educational attainment [29]. Public health campaigns need to address this gap in both understanding and behaviour changing [30].There is an ongoing major need for further investigations in the field of alcohol consumption during pregnancy, because in Italy alone, every year, about 3,7 ‰ newborn children suffer from FAS, and even more are those born with symptoms of fetal alcohol spectrum disorder. We performed this cross-sectional study as a consecutive cohort of delivering pregnants. No previous detailed information was gathered on lifestyle behaviours including alcohol intake, smoking, diet, exercise and infant feeding intention so we did not actually know the patients’ declarations about alcohol consumption.
ACKNOWLEDGMENTS
- This study was funded by Verbano-Cusio-Ossola Community Foundation.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML