-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Health Science
p-ISSN: 2166-5966 e-ISSN: 2166-5990
2015; 5(5): 81-88
doi:10.5923/j.health.20150505.01

Hand Grip Strength in Elderly Patients with Chronic Illnesses: A Case Control Study
Olabisi A. Akinwande1, Olufemi A. Adegbesan2, Joseph F. Babalola2, Abiola A. Atowoju1, Chidozie E. Mbada3
1Department of Physiotherapy, University College Hospital, Ibadan, Nigeria
2Department of Human Kinetics and Health Education, Faculty of Education, University of Ibadan, Ibadan, Nigeria
3Department of Medical Rehabilitation, College of Health Sciences, Obafemi Awolowo University, Ile - Ife, Nigeria
Correspondence to: Chidozie E. Mbada, Department of Medical Rehabilitation, College of Health Sciences, Obafemi Awolowo University, Ile - Ife, Nigeria.
| Email: |  |
Copyright © 2015 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Background: Weak Handgrip Strength (HGS) is associated with poor functional performance capacity and frailty especially among older adults. This study compared HGS among elderly individuals with chronic illnesses and healthy controls. Methods: 176 (88 patients and 88 healthy controls) participants were purposively recruited for this case-control study. World Health Organization’s definition of old adult (i.e. 65 years and older) was adopted for the inclusion criteria. A handheld dynamometer was used to assess HGS. Anthropometric parameters were measured following standard procedures. Clinical bio-data of the patients were gleaned from case notes. Data were analyzed using descriptive and inferential statistics. Alpha level was set at p<0.05. Results: The participants’ ages ranged between 65 and 84 years. The patient group had significant higher anthropometric measures (p<0.05). The controls had higher Dominant HGS (DHGS) (22.8 ± 7.12 vs. 26.5 ± 4.76 Kgf; p=0.001) and Non-Dominant HGS (NDHGS) (22.0 ± 6.21 vs. 25.4 ± 4.65 Kgf; p=0.00) respectively. The control group ‘males’ had significantly higher DHGS and NDHGS (p<0.05) while the patients’ group females had the least DHGS and NDHGS scores (p<0.05). Patients with neuromuscular conditions or multimorbidity had significantly lower DHGS and NDHGS (p<0.05). There was significant association between level of HGS and each of sex, age group and BMI classification (p<0.05). There were higher number of participants with weak and intermediate HGS in the patient group compared with controls (p=0.001). Regression analysis revealed that age, weight, height, BMI and duration of illness were significant predictors of DHGS and NDHGS respectively (p<0.05). The variability of the predictive model for DHGS and NDHGS was 37.7% and 52.2% respectively. (R2 =52.2). Similarly, only ‘age (p=0.011) and ‘duration of illness (p=0.001) were significant predictors of dominant HGS. Conclusions: Elderly individuals with chronic illnesses have poorer HGS than healthy controls. Having a neuromuscular conditions or multimorbidity significantly impaired HGS than illnesses of musculoskeletal origin. Age, gender and anthropometric measures significantly influence HGS. Age, weight, height, BMI and duration of illness seems to be moderate predictors of HGS but not without significant errors.
Keywords: Handgrip strength, Elderly, Chronic illness, Multimorbidity
Cite this paper: Olabisi A. Akinwande, Olufemi A. Adegbesan, Joseph F. Babalola, Abiola A. Atowoju, Chidozie E. Mbada, Hand Grip Strength in Elderly Patients with Chronic Illnesses: A Case Control Study, Journal of Health Science, Vol. 5 No. 5, 2015, pp. 81-88. doi: 10.5923/j.health.20150505.01.
1. Introduction
- Weak Handgrip Strength (HGS) is associated with poor functional performance capacity and frailty especially among older adults [1-3]. Therefore, HGS has become an important surrogate measure of total body strength and a biomarker of other systems such as the endocrine system [4]. Consequently, HGS assessment is a common procedure among clinicians and researchers to measure functional integrity of the upper extremity [5-7], diagnose impairment of hand muscle power, and it is also a rehabilitation outcome tool [8]. Hand grip strength is extremely important to carry out daily activities, such as holding objects, carrying out domestic tasks, and self-care activities. There is a gamut of physiologic and pathologic events that may negatively impact on HGS and consequently on functional performance. Of the physiological factors, studies have identified that HGS has a curvilinear relationship with age [1-2, 9]. The inverse relationship between HGS and aging process significantly influences the living activities of elderly [10, 11]. As a result, low HGS is linked with premature mortality in middle-aged and elderly subjects [12-14]. Therefore, HGS in the elderly is a valid predictor of functional limitations and disability and it is associated with disability and other health complications [12, 13].Consequent to the foregoing, assessment of HGS performance in elderly is of high clinical importance. Rantanen et al [15] noted that there are different measurement methods, which may be used to assess maximum HGS. These include: cable tensiometer, handheld dynamometer, modified sphygmomanometer and subjectively using the examiner’s hand pressure. However, the dynamometry method is the most widely used in the HGS assessment method in the clinical settings as well as for research purposes based on its good psychometric properties [15-17]. Although, the Jamar dynamometer is reported to give the most accurate and acceptable measures of HGS, nonetheless, recent advancements in technology, have heralded many modified and simpler types of dynamometers such as digital dynamometers, hydraulic dynamometers, the Lode grip dynamometer, and the Takei Kiki Kogyo dynamometer among others. These new dynamometers have been reported to have good psychometric properties, cost-effective and also with high clinical research usability [5-7, 10, 18]. Thus, HGS measured by dynamometry has good correlation with upper limb functional level and general health status, and it is widely used to select therapeutic procedures and for functional rehabilitation follow-up [10, 18, 19]. Traditionally, HGS tests have been used in rehabilitation to evaluate the physical fitness of the upper limbs, by measuring the strength of hand and forearm muscles in health and disease. HGS assessments have been carried out in chronic illnesses affecting upper extremities such as rheumatoid arthritis, carpal tunnel syndrome, lateral epicondylitis, stroke, traumatic injuries and neuromuscular diseases. Results of HGS assessments in these patients are used in the clinical and rehabilitation settings to determine the extent of injury or disease process and also to measure the progress of rehabilitation [19]. On the other hand, some studies have established normative values for HGS with which to compare scores obtained from patients’ groups [5-7, 10, 18]. However, the generalizability of normative data on HGS are limited by methodological differences with respect to equipment and research protocols and lack of ubiquitous cut-points. In addition, other factors such as gender, age, dominance, evaluation time, body positioning and anthropometric characteristics may limit the external validity of normative values of HGS. Despite the foregoing, HGS, especially in the elderly represent a surrogate measure and valid predictor of functional limitations, frailty, incident mobility limitations, disability and it is associated with other health complications [12, 13]. In addition, aging and development of chronic illnesses are mutually related events. However, the toll of chronic illnesses on HGS among older adults needs to be investigated. Therefore, it is necessary to determine the contribution of chronic illnesses on HGS among older adults. The objective of this study was to compare HGS between elderly individuals with chronic illnesses and healthy controls; and also to establish a prediction model for HGS based on age, anthropometric parameters and duration of illnesses.
2. Methods
- This study adopted a case-control research design. World Health Organization’s definition of old adult (i.e. 65 years and older) was adopted for the inclusion criteria. 176 (88 patients and 88 healthy controls) participants were purposively recruited for the study. The patients’ group were elderly individuals receiving physiotherapy at the Geriatric Physiotherapy Outpatient Clinic, University College Hospital, Ibadan, Nigeria. The patients were recruited based on physician’s diagnosis of musculoskeletal/orthopedics conditions (including musculoskeletal pain, cervical spondylosis and osteoporosis) and neurological conditions (cervical myelopathy etc.). Multimorbidity was operationalized in this study as having co-occurrence of musculoskeletal and neurological conditions within the same person [20]. The healthy controls in this study were elderly individuals who reported no history of musculoskeletal joint disease, neurological disorder or injury to the upper extremity, in addition to having no obvious restriction of movement in the upper extremities. Handgrip Strength Assessment Handgrip strength was measured using a standard handheld dynamometer (Haoyue, China) according to the standard protocol of the American Society of Hand Therapists (ASHT) recommendations [21]. The HGS assessment protocol involved asking the participant to sit on a chair with shoulder adducted and neutrally rotated and elbow in 90° flexion with no radioulnar deviation. The participant was instructed to squeeze the handle maximally and to sustain this for 3–5 seconds. Each participant performed three maximum attempts with one-minute rest between attempts for each dominant and non-dominant HGS assessment in order to reduce minimize fatigue effects. No verbal encouragements were given during the assessment. The mean value of these trials was used in the final analysis. Results were recorded in kilogram force (kgf). Dominant hand was determined as by asking about the preferred hand for daily activities like cleaning, sweeping, making home chores and eating [22]. Anthropometric parameters assessed in this study include height, weight and Body Mass Index. Body weight was measured using a portable weighing scale to the nearest 0.1 kg. Height was measured with a height meter to the nearest 0.1 cm. Body mass index (BMI) was computed as the ratio of weight and height squared. Data were also obtained on socio-demographics of all participants and clinical characteristics of the patients. Statistical analysisData were summarized using descriptive statistics of frequency, mean and standard deviation. Inferential statistics of Chi-Square, Independent t-test, One-Way ANOVA and multiple regression analysis were used. The SPSS version 16.0 programme (SPSS Inc., Chicago, IL, USA) for Windows was used for statistical analysis. Apha level was set at p<0.05. ComputationHand Grip Strength Classification-In men, a grip strength of 26–32 kgf was classified as “intermediate” and less than 26 kgf as “weak”; while >32 kgf was classified as normal. In women, a grip strength of 16–20 kg was classified as “intermediate” and less than 16 kg as “weak”; while > 20 kg was classified as normal [3, 23].
3. Results
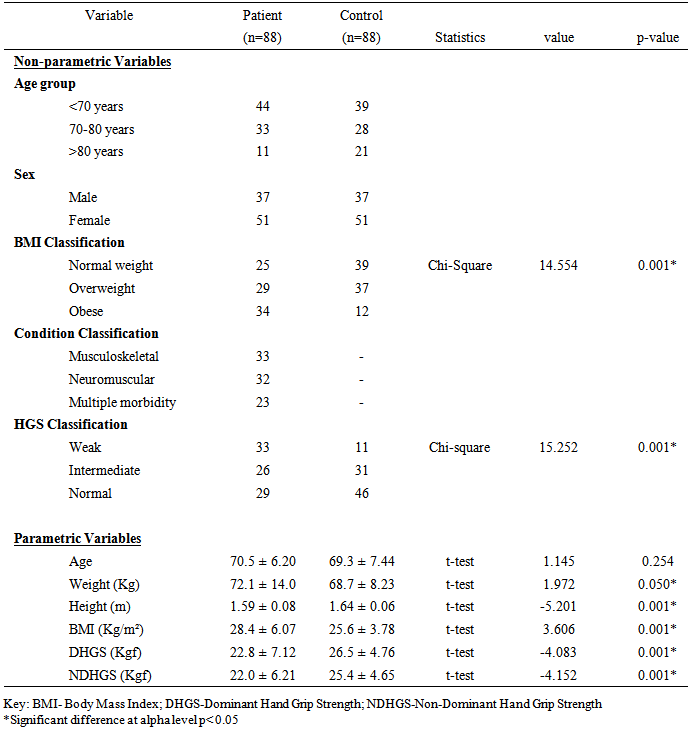
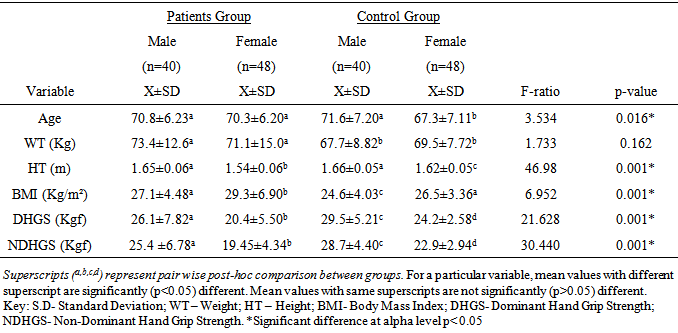
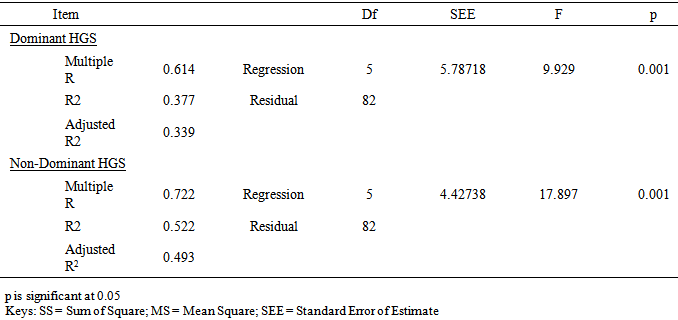
- The participants’ ages ranged between 65 and 84 years. All the participants in this study were right hand dominant (100%). The socio-demographics, anthropometric parameters, clinical characteristics and HGS of the participants by group are presented in table 1. The result shows that the patient group had significant higher anthropometric measures (weight, height and BMI) (p<0.05). However, the controls had higher Dominant HGS (DHGS) (22.8 ± 7.12 vs. 26.5 ± 4.76 Kgf; p = 0.001) and Non-Dominant HGS (NDHGS) (22.0 ± 6.21 vs. 25.4 ± 4.65 Kgf; p=0.00) respectively. The result of the One-Way ANOVA and LSD post-hoc comparison of the anthropometric characteristics and HGS of participants by group and sex is presented in table 2. The result showed that there were significant gender differences in anthropometric variables by group and by sex (p<0.05). The female patients’ group had significantly higher BMI (p<0.05), however, the control group males had significantly higher DHGS and NDHGS (p<0.05) followed by the patients’ group males. The female patients’ group had the least DHGS and NDHGS scores (p<0.05).
|
|
|
|
|
4. Discussion
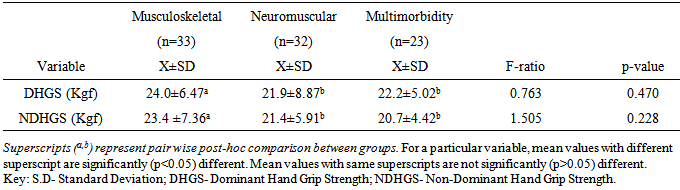
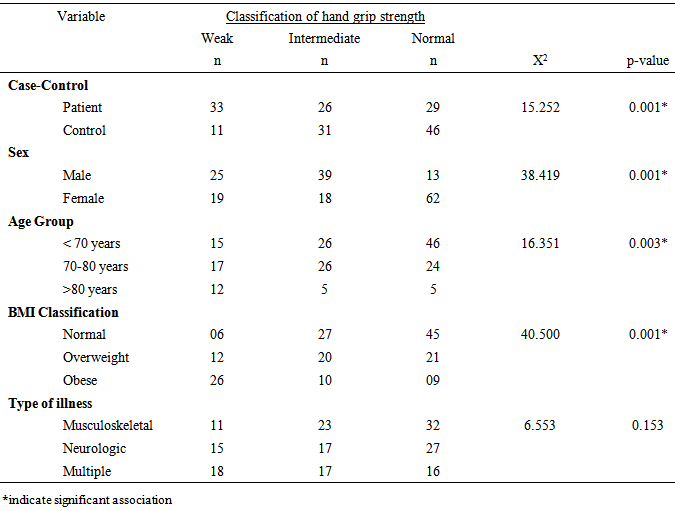
- Most studies among the elderly individuals seems not to take into account the mutual effects of the inter-relationships between chronic illnesses and aging on HGS. Therefore, it is necessary to determine the contribution of chronic illnesses on HGS among older adults. This study compared HGS among elderly individuals with chronic illnesses and healthy controls. The finding of the study showed that the patient group had significant higher anthropometric measures and BMI distribution of overweight and obesity (especially among the female patients). The patients’ group had significantly lower HGS compared with the healthy controls with poorer grip strength among the female patients. This finding is consistent with earlier studies that have reported reduced HGS in elderly patients with chronic illnesses [24-26]. The finding of this study, is consistent with previous reports showing that elderly female patients had significantly higher BMI [27-29] and lower HGS for the dominant and non-dominant hand compared with their age-matched male counterparts [4]. However, in addition to the specific effect of morbidity on HGS, it is adduced that the significantly higher BMI among the patient group could have contributed to the lower HGS among them.This study found significant association between level of HGS and each of sex, age group and BMI classification. Literature is replete with studies showing strong correlation between HGS and anthropometric traits [30]. Specifically, higher weight and BMI are associated with reduced HGS without gender bias [31, 32]. Elderly patients with chronic illnesses have higher risk of overweight and obesity owing to morbidity-related decreased mobility, functional limitation and sedentariness [33]. Increase in BMI could have accounted for the reduced HGS observed among the elderly patients in this study. This study further reveals that patients with neuromuscular conditions or multimorbidity had significantly lower HGS. The finding of this study further revealed that having a neuromuscular condition or multimorbidity status led to significant lower HGS compared with having a musculoskeletal condition. Apparently, neuromuscular conditions have direct impact on the nerves which grossly limit the ability of the muscles they supply to contract and move the fingers to make a grip. However, for musculoskeletal conditions, the nerves are usually intact, but movement restriction may only be due to pain, stiffness etc. which patients may still try to work against, with much effort and endurance. It appears that more studies have investigated the influence of orthopedic or musculoskeletal conditions on HGS [34] than non-musculoskeletal conditions. However, there is accumulating reports that suggest that HGS could be influenced by neuromuscular system, endocrine or other physiologic systems [35]. It is hypothesized that HGS is associated with multiple physiological systems and as such, impaired or reduced HGS could be associated with insulin resistance and hyperthyroidism and increased interleukin-6 or reduced insulin-like growth factor I (IGF-I) among other covert mechanisms. The finding of this study somewhat contrasts the study by Cheung et al [26] who reported that a number of chronic diseases influence HGS [36], where co-occurrence of two or more chronic diseases or multimorbidity was significantly associated with lower HGS compared with a single disease condition (musculoskeletal or neuromuscular). The present study found no significant difference in HGS between elderly patients with neuromuscular conditions and those with multimorbidities. In addition, this study found a weak to moderate correlation between the physical characteristics and HGS among elderly patients with chronic illnesses, but not with controls.The regression prediction models in this study suggest age, weight, height, BMI and duration of illness could account for DHGS and NDHGS. The variability of the predictive model for DHGS and NDHGS was 37.7% and 52.2% respectively. However, the variability level of 37.7% and 52.2% for DHGS and NDHGS respectively was from low to moderate and it thus indicate that the use of the regression prediction models derived from this study for HGS could not be without significant errors. Considering that there are other important variables that determines HGS in elderly which were not assessed in the current study. For example, nutrition of the elderly people is a significant predictor of HGS [37, 38] as well as lower socio-economic class [39-41], reduced physical activity level [41], and reduced cognition [12] among others. Therefore, the results of this study seem to shed more light on the inter-relationships between chronic illnesses and aging on HGS in the elderly. In addition, this study revealed the pattern and association between HGS and socio-demographic and anthropometric variables among elderly individuals. From this this study, routine evaluation of HGS among older adults is recommended as it may have both diagnostic and prognostic values. Also, case-control approach for the evaluation of HGS among older adult may be helpful to identify the elderly with weak and intermediate HGS who may benefit from strength improvement interventions. Furthermore, researchers and clinicians should take cognizance of the impact of anthropometric parameters (especially BMI) and type and duration of illness on HGS in the elderly. However, further studies are needed to establish normative database for HGS in the elderly and also validate the predictive model proposed in this study. The potential limitations of this study include, the inability to ascertain the health status of the control participants as the state of health was based on self-report and also eliminate the influence of personal motivation in HGS tests. Also, the clinical status of the patients’ group was based on data recorded in their case notes and interviews. In addition, a heterogeneous sample of elderly patients who were grouped based on similarities of their medical conditions rather than being homogenous were recruited in this study. Therefore, future studies comparing homogenous elderly patient samples are needed to validate the finding of this study.
5. Conclusions
- Elderly individuals with chronic illnesses have poorer HGS than healthy controls. Having a neuromuscular conditions or multimorbidity significantly impaired HGS than illnesses of musculoskeletal origin. Age, gender and anthropometric measures significantly influence HGS. Age, weight, height, BMI and duration of illness seems to be moderate predictors of HGS but not without significant errors.
ACKNOWLEDGEMENTS
- The authors wish to thank the participants for volunteering in this study. Also, the co-operation of all clinical and clerical staff of the Geriatric Physiotherapy Outpatient Clinic is highly appreciated.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML