-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Health Science
p-ISSN: 2166-5966 e-ISSN: 2166-5990
2014; 4(4): 99-104
doi:10.5923/j.health.20140404.04
Drug Utilization Pattern in Pregnancy in a Tertiary Hospital in Sokoto, North West
Abubakar K.1, Abdulkadir R.2, Abubakar S. B.3, Jimoh A. O.4, Ugwah-Oguejiofor J. C.1, Danzaki A. M.5
1Department of Pharmacology and Toxicology, Faculty of Pharmaceutical Sciences, Usmanu Danfodiyo University Sokoto, Nigeria
2Pharmacy Department National Ear Care Centre Kaduna, Nigeria
3Department of Haematology and Pathology, Usmanu Danfodiyo University Teaching Hospital, Sokoto, Nigeria
4Department of Pharmacology and Therapeutics college of Health Sciences, Usmanu Danfodiyo University, Sokoto
5Department of Clinical Pharmacy and Pharmacy Practice, Faculty of Pharmaceutical Sciences Usmanu Danfodiyo University Sokoto
Correspondence to: Abubakar K., Department of Pharmacology and Toxicology, Faculty of Pharmaceutical Sciences, Usmanu Danfodiyo University Sokoto, Nigeria.
| Email: |  |
Copyright © 2014 Scientific & Academic Publishing. All Rights Reserved.
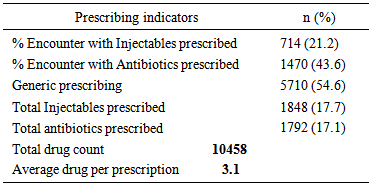
Background: The state of pregnancy requires that all medications should be prescribed with specific caution. The physiological state of pregnancy has an effect on the pharmacokinetics of drugs administered, with attendant risk on the developing foetus. Therefore categorization of drugs according to their pregnancy risk should be taken into consideration before a drug is prescribed for pregnant women. Objective: To evaluate the prescription behaviour of physicians at the obstetrics and gynaecology department of a tertiary health institution in North West Nigeria. Methodology: This was a one year retrospective study, data was collected by way of a structured questionnaire and convenient sampling method was employed. Case notes of women who attended the clinic and receive a prescription were selected, 3374 prescriptions were selected based on exclusion and inclusion criteria. Results: Majority of the patients in the study (57.3%) were within the reproductive age (25-34%). 73% of the study subjects were multigravid and in their third trimester (41.9%).T he average drug per prescription was 3.1, encounter with antibiotics was 43.6% while encounter with injections was 21.2%. The third trimester accounts for most of the prescriptions (48.8%), and antimalarials were the most prescribed group of drugs 36.6%. Amoxicillin was the most prescribed antibiotic and arthemeter lumefantrine was the most prescribed antimalarial agent. Conclusion: The prescription behaviour of prescribers in the hospital under study is encouraging since most core INRUD prescribing indicators were been adhered to. However, the issue of polypharmacy need to be addressed.
Keywords: Pregnancy, Trimester, Artemether
Cite this paper: Abubakar K., Abdulkadir R., Abubakar S. B., Jimoh A. O., Ugwah-Oguejiofor J. C., Danzaki A. M., Drug Utilization Pattern in Pregnancy in a Tertiary Hospital in Sokoto, North West, Journal of Health Science, Vol. 4 No. 4, 2014, pp. 99-104. doi: 10.5923/j.health.20140404.04.
Article Outline
1. Introduction
- The use of drugs in pregnancy is an issue of great concern to the patients and prescribers alike. The Thalidomide incidence of the 1960’s and the terratogenic effects that was discovered related to Diethylstilboesterol in 1971 [1] are a few instance of the dangers which prescription drugs may pose to pregnant subjects. Pregnancy is associated with changes in the physiological, psychological and psychosocial aspects of a woman’s life. Drug pharmacokinetic parameters are usually affected by the type of drugs administered in pregnancy, most administered drug passes the placental barrier, and depending on the stage of pregnancy, the life of the foetus may be threatened. Therefore, special consideration needs to be given to drug prescribing in pregnancy [2]. Safe use of drugs in pregnancy is a responsibility of all members of the healthcare team, nevertheless, the bulk of this responsibility rests on the Physicians shoulder. Medicines are responsible for up to 1% of reported cases of congenital malformations [3], therefore safety of prescribed drugs to the mother and her unborn baby has to be considered during drug prescribing. Medicine prescription in pregnancy and lactation would therefore require good knowledge of terratogenecity, foetal and neonatal effects that are associated with the medicines under consideration [4].Generally, drug treatment should be avoided during pregnancy [4], but sometimes this becomes inevitable because some patients are already receiving treatment for some chronic diseases such as Diabetes mellitus, asthma or hypertension before the onset of the pregnancy. Such patients will have to continue their treatment; hence the need for careful selection of the appropriate drug for their condition [5]. Animal studies and randomised clinical trials tend to exclude pregnant subjects during experimentation, therefore safety of most drugs in pregnancy is only known through post market surveillance and through data from agencies such as Food and drug administration (FDA) in the United State of America and the National agency for the control of food and drugs (NAFDAC), Nigeria. Supplementary drugs such as iron, folic acid, calcium, ascorbic acid and vitamin B complex are commonly prescribed to provide for the increase demand of these essential nutrients during pregnancy. In addition analgesics such as paracetamol, expectorants anti emetics, antacids and antibiotics for urinary tract infections (UTI) are frequently prescribed [6]. Drug utilization studies (Pharmacoepidemiology) is the study of the utilization and effects/side effects of drugs in large numbers of people with the purpose of supporting rational and cost- effective use of drugs in the population thereby improving health outcome. Pharmacoepidemiology may be drug-oriented, emphasizing the safety and effectiveness of individual drugs or group of drugs, or utilization oriented aiming to improve the quality of drug therapy through educational intervention [7]. The present study aims to unearth the pattern of drug utilization among pregnant women in Sokoto, north western Nigeria, by obtaining data though case folders of patients attending a tertiary health facility in this city. The scope will include evaluating the present practices in prescribing and future trends of drug usage and appropriateness of prescriptions [8]. It is therefore pertinent to assess the drug utilization pattern in pregnancy to know the extent to which good prescribing practices in these ‘special population’ are been adhered to. Previous studies of drug utilization pattern in pregnant women include studies in some health centres in India [9, 10], south west Nigeria, [4] to mention just a few.
2. Method
- This was a one year retrospective study (1st January, to 31st December, 2012). Data was collected by way of a structured questionnaire. Convenient sampling method was employed. Case notes of pregnant women that attended the clinic and receive a prescription were selected and used for the study. The questionnaire design includes patient’s socio- demographic data, obstetric history and medication profile. The pregnant women and their corresponding drug use were classified according to trimester and compared to available recommended guidelines. Following the use of some exclusion criteria such as legibility of writing, prescription without signature and other parameters of a complete prescription, 3374 prescriptions were selected for this study.
2.1. Ethics Committee Approval
- The Institutional Ethics Committee permission was taken prior to initiation of the study.Written Informed Consent was taken from all the pregnant women before their prescriptions were analyzed.
2.2. Hospital Background
- The study was conducted at the Department of obstetrics and gynaecology, Usmanu Danfodiyo University Teaching Hospital, Sokoto. The hospital is a tertiary care hospital which receives referrals from other private clinics, hospitals and general Physicians. The hospitals antenatal department receives between 100-120 pregnant women daily. The clinic operates from Mondays to Fridays, three days for follow-ups and two days for fresh bookings. The clinics includes gynaecological emergency and normal check-up, patients without complains were only asked to continue taking their routine drugs, while patients with complains will have to see the physician and probably get a prescription. Most of the women attending this center for antenatal services were married and not gainfully employed.
2.3. Prescribing Practices
- The department has about eleven consultants, five senior registrars, twenty two registrars and nine house officers. Pregnant women attending the clinic are examined by any of these doctors and in most cases handed over a hand-written prescription. The Pregnant women then obtain their drugs either from the hospital Pharmacy or some Pharmaceutical premise outside the hospital.
|
2.4. Statistical Analysis
- Data collected was entered into a spread sheet and analysed using descriptive statistics. Data was further analysed using the statistical package for social science students (SPSS) 17.0. The WHO/INRUD methods of determining core prescribing indicators were employed. The average number of medicines per encounter was calculated by dividing the total number of drugs by the number of encounters. Percentage encounter with generic name, percentage encounter with antibiotics and percentage of encounter with injections were determined by dividing the total number of occurrence by the total number of event, respectively and multiplying by 100.
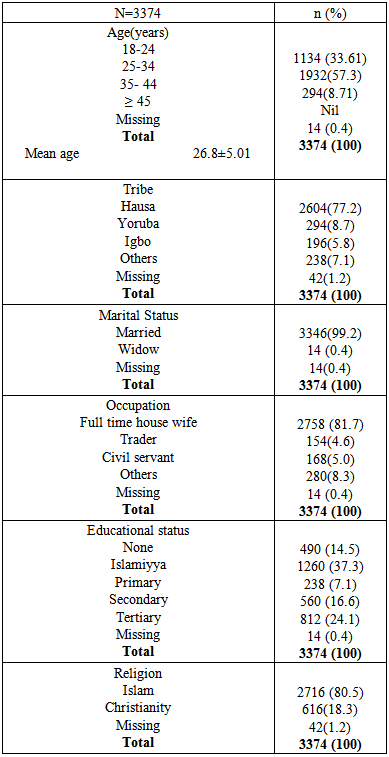
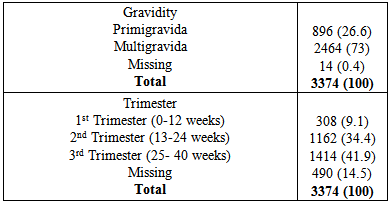
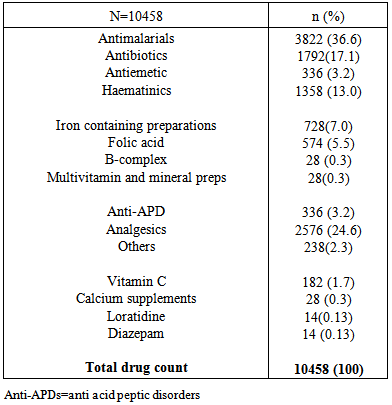
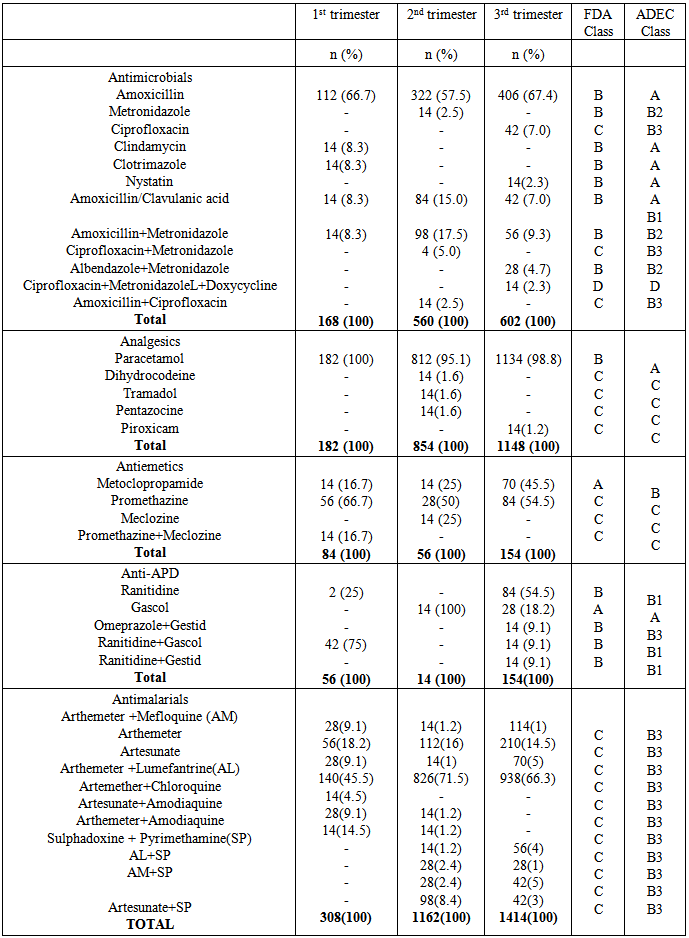
3. Results
|
|
|
|
4. Discussion
- Significant findings: The research was able to establish that women in their third trimesters attend the antenatal clinics more than those in the first and second trimesters. Injectables drugs prescription doubled the recommended WHO standard in this study. Antimalarial drugs were the most prescribed drugs in this study. Nigerian governments’ recommendation of the use of ACTS’ was fully implemented on the drug in the study. Finally there was full consideration of the FDA and ADEC categorization of the risk status of drugs to pregnant women.The mean age of the women in this study was 26.8± 5.01; this is in agreement with the findings of [11, 12]. Majority of the women in this study were married (81.7%), this finding could be attributed to the socio- cultural and religious background of the people whereby giving birth out of wedlock is abhorred. Multigravid women were the majority in this study (73%); this could be due to the increase awareness from organizations that are campaigning to ensure reduced maternal mortality and safe motherhood. Pregnant women in their third trimester formed the bulk of the patients in the study. Similar findings have been reported previously in India and eastern Sudan where majority of the pregnant women were in their third trimesters [13, 11]. Percentage encounter with Injectables was 21.2% which was higher than the acceptable range of 10% or less, the increased number could be due to the problem of hyperemesis gravidarium especially in the first trimester and in mostly primigravid patients. [14] reported a study where encounter with Injectables was less (2.4%), similarly [15] reported 40% encounter with Injectables in a study, though the result was attributed to seasonal changes. Percentage encounter with antibiotics was found to be 43.6%, this is higher than the recommended value of 20%. Antibiotics are usually prescribed with caution due to the problem of drug resistance, bearing this in mind the higher percentage of antibiotics prescribed in the study could be due to opportunistic infections, respiratory and urinary tract infection which occur commonly among pregnant women. Similar result (43.5%) was reported by [16]. In contrast to this finding was the report of [18] who reported a value less than the recommended.The average drug per prescription was 3.1; this is higher than the WHO range of 2 or less. Polypharmacy in some instance becomes necessary especially when the patient has some co-morbid conditions associated with the pregnancy. Moreover, most pregnant women take haematinics and vitamins such as Iron preparations, folic acid, ascorbic acid and vitamin B complex tablets. Similar values have been reported in Nigeria [17, 18] Majority of the patients were prescribed three drugs (48.6%), followed by four drugs (25.3%), only 6 patients had up to six drugs, which was probably due to their presentation with urinary tract infection (UTI). Amoxicillin was the most prescribed antibiotic (70.7%). Amoxicillin a broad spectrum antibiotic has a track record of safety in pregnant women, according to a report on the use of amoxicillin among pregnant women in Denmark; it was found not to increase risk of adverse pregnancy outcome associated with amoxicillin exposure during pregnancy [19]. Paracetamol was the most prescribed analgesic throughout the three stages of pregnancy (>95%), this may be due to its affordability, tolerability and lack of the adverse effect of the NSAIDS. Similar studies where paracetamol was the major analgesic antinflammatory agent includes [4, 9, 10, and 20]. 54.6% of all the drugs used were prescribed using their generic names, the WHO recommended 100% of all drugs be prescribed using generic names, this value was less than that reported by [14]; (72.8%.). Similarly [9] reported that 21.5% as the percentage of drugs prescribed using their generic names. Therefore, the practice of using brand names should be discouraged as this causes economic hardship on the patients. Antimalarials were the most prescribed (36.6%), arthemeter and Lumefantrine combination was the most prescribed of the artemisinin-based combination (ACTs) 56%. The ACTs are at present the bane of malaria treatment in Nigeria. The government of Nigeria ban the use of chloroquine in the management of malaria due to the increase incidence of treatment failure that have been observed by many physicians. Though, it is still a subject of debate in some quotas that chloroquine is still the best antimalarial agent. Personal interview with some Physicians and patients still suggest that the use of chloroquine in Malaria treatment is far from being over. Studies reporting the use of ACTs in pregnancy includes [21], they reported the prevalence of use of ACTs throughout the three stages of pregnancy. But caution need to be exercised in the first trimester as data regarding the safety of the drug during this period is still lacking. Similarly, studies carried out in South east Nigeria [22] reveals a 43.6% prescription of ACTs, in contrast another study in Osogbo and Calabar Nigeria in 2005 and 2007 reported 18.6% and 3% respectively [23,24] Antiemetics were mostly prescribed during the first trimester to control hyperemesis gravidarium also called “morning sickness”. The most prescribed in our study was promethazine (38.4%). The FDA categorize promethazine under C, therefore it is advised to be used only when the risk outweigh the benefit.The United states food and drug administration (FDA) and the Australian drug evaluation committee (ADEC) classification of drugs was used to evaluate the risk categorization of the drugs prescribed in this study. Doxycycline was the only drug belonging to category “D”. Drugs in this category according to the FDA and ADEC are classified as drugs with potential risk although; they can be used when the benefit outweighs the risk. All other drugs in the study are within the safe zone, that is A-C (FDA) and (A-C, B1-B3), ADEC categorizations.
5. Conclusions
- The study shows considerable medication use during pregnancy, the prescription behaviour of physicians in the hospital under study is quite encouraging. WHO/INRUD indicators of good prescription behaviour were adhered to. Nevertheless, more need to be done in the areas of polypharmacy since this index was observed to be higher than the standard. Contraindicated medicines were absent in this study. No woman was prescribed Category X drug. Pregnant women with infectious diseases and malaria were treated with the appropriate drugs considering the risk benefit ratio. Most of the drugs were prescribed in generics and not in brand names. Thus, prescribing pattern observed in our study sets a fine example of prescribing behaviour.
ACKNOWLEDGEMENTS
- The authors wish to express their sincere appreciation to the staff of the Department of Obstetrics and gynaecology Usmanu Danfodiyo University Sokoto for their support in ensuring the success of this research work.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML