-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Health Science
p-ISSN: 2166-5966 e-ISSN: 2166-5990
2014; 4(1): 18-23
doi:10.5923/j.health.20140401.04

A Study on Erioglossum rubiginosum for Evaluation of Biological Properties
S. M. Masud Rana, Md. Mustahsan Billah, Santanu Barua, Md. Mizanur Rahman Moghal, Golam Sarwar Raju, Md. Mahmodul Islam
Department of Pharmacy, Noakhali Science and Technology University, Sonapur, Noakhali-3814, Bangladesh
Correspondence to: Md. Mustahsan Billah, Department of Pharmacy, Noakhali Science and Technology University, Sonapur, Noakhali-3814, Bangladesh.
| Email: |  |
Copyright © 2014 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Objectives: Present study was carried outto evaluate Pharmacological (cytotoxic and antimicrobial activities), phytochemical and physicochemical properties of Erioglossum rubiginossum leaves, an evergreen plant, belonging to the family Sapindaceae. Methods: Crude methanol extracts and fraction of the leaves of E. rubiginossum were used for brine shrimp lethality bioassay to identify cytotoxic potential of the extractives compared to Vincristine sulphate. The crude extract (methanol extract and fractions) of E. rubiginossum was screened for their antimicrobial activity against a wide range of bacteria and fungi by disc diffusion method. The phytochemical evaluation was carried out by qualitative analysis. Physicochemical properties was determined according to method (Anonymous 1968) described in British Pharmacopeia. Result: In brine shrimp lethality bioassay, the carbon tetra chloride soluble fraction possessed the highest cytotoxic activity with LC50 value of 11.11±0.04µg/ml. The LC50 values of fractions were found to be 11.11 to 90.05µg/ml compared to standard Vincristine sulphate (0.451µg/ml). From these results, it can be well predicted that the leaves of E. rubiginossum possesslow cytotoxic property. In disc diffusion assay, the fractions of E. rubiginosum possessed zone of inhibition ranging from 3.0 to 14.0 mm. Among them, the chloroform soluble fraction had shown highest zone of inhibition 14.0 mm against Salmonella paratyphi. The phytochemical evaluation showed presence of alkaloids, flavonoids, phenols, saponins and carbohydrate. Various physicochemical parameters like moisture content, total ash value, acid soluble ash value, water soluble ash value, alcohol soluble extractive value and water soluble extractive value were to be found 7.20%, 21.00%, 23.00%, 8.50%, 1.00% and 0.50% respectively. Conclusion: Further investigations are required for isolation of active compound that are responsible for antimicrobial activity and cytotoxicity along with their mechanism.
Keywords: Erioglossum rubiginosum, Brine shrimp lethality, Zone of inhibition, Phytochemicals, Physicochemical
Cite this paper: S. M. Masud Rana, Md. Mustahsan Billah, Santanu Barua, Md. Mizanur Rahman Moghal, Golam Sarwar Raju, Md. Mahmodul Islam, A Study on Erioglossum rubiginosum for Evaluation of Biological Properties, Journal of Health Science, Vol. 4 No. 1, 2014, pp. 18-23. doi: 10.5923/j.health.20140401.04.
Article Outline
1. Introduction
- The plant under research Erioglossum rubiginosum belongs to the family Sapindaceae. The common name of the plant is Kalayo. Commonly using part of the plant is roots, barks and leaves. Previous study had shown that major components of flower essential oil were nerolidol (34.8%), palmitic acid (13.2%), and farnesol (10.0%). Fruit essential oil yielded palmitic acid (66.1%), myristic acid (10.0%), and linolenic acid (5.5%) [1]. Methanolic fraction isolated a tetrasaccharide derivative of farnesol named rubiginoside along with known triterpenoidsaponins [2]. The plant is commonly used for the treatment of leprosy [3]. This plant is extensively used as folkloric medicine such as roots are used as astringent, leaves and fruits are used for the treatment of fever and poultice [4, 5]. Recent investigation had shown the leaves of the plant could be used as a natural source of membrane stabilizers [6]. Another recent investigation had proved that the leaves of the plant possess antioxidant and thrombolytic [7]. CNS depressant activity was found significantly by this plant [8].However, indiscriminate use of medication has emerged problems, driving to the development of resistance as well as chemical residue and toxicity problems [9]. To do literature survey, the information of medicinal property of E. rubiginosum, leaves is still lacking. The native use of E. rubiginosum as medicament, prompted us to research the phytochemical analysis, cytotoxic, antimicrobial activity and physicochemical properties that has not been explored so far. Therefore, we selected E. rubiginosum, leaves as a part of our ongoing research to expose its medicinal property in terms of its cytotoxic, antimicrobial activities, phytochemical and physicochemical properties.
2. Materials and Methods
2.1. Plant Material
- The leaves of E. rubiginosum was collected from National Botanical Garden, Mirpur, Dhaka. The plant was identified by the taxonomist of Bangladesh National Herbarium, Mirpur, Dhaka, Bangladesh and a voucher specimen has been deposited in the herbarium unit (Accession no DACB 38566).After collection, the leaves of the plant were thoroughly washed with water. The leaves were sun dried for several days and then oven dried for 24 hours at considerably low temperature for better grinding. After drying, the total leaves were coarsely fine-grained (120g) and extracted by dissolving with methanol (500mL) for fifteen days concomitant occasional shaking and stirring. The sediments were filtered and then dried at 40°C during a water bathtub. The solvent was utterly removed by filtering with Whatmanpaper (Bibby RE200, Sterilin Ltd., UK) and obtained dried crude methanol extract (blakish-brown). An aliquot (5g) of the concentrated methanol extract was fractionated by modified Kupchan partition protocol [10] and the resultant partitionates were evaporated to dryness with rotary evaporator to yield pet ether (PESF, 1.5g), carbon tetrachloride (CTCSF, 1.5g), chloroform (CSF, 1g) and aqueous (AQSF, 0.5g) soluble fractions. The residues were then stored in the refrigerator until further use.
2.2. Brine Shrimp Lethality Bioassay
- Brine shrimp lethality bioassay was used for probable cytotoxic action according to Meyer et al [11]. Ten brine shrimp matured shrimps were applied to each of all experimental vials and control vial. The mortality of brine shrimp was observed after 24 hours of treatment for each of the concentrations. An approximate linear correlation was observed, when logarithm of concentration versus percentage of mortality was plotted and the values of LC50 were calculated by probit analysis method described by Finney [12]. Vincristine sulphate was used as a positive control.
2.3. Antimicrobial Screening
2.3.1. Test Organisms
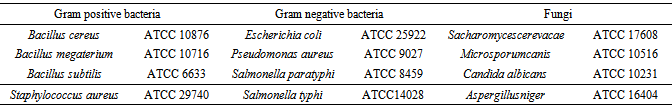
- Four strains of Gram-positive bacteria, Gram-negative bacteria and four strains of fungi were used to evaluate the antimicrobial activity (Table 1). The organisms were sub cultured in nutrient broth and nutrient agar. They were collected from the Department of Microbiology, Chittagong Veterinary and Animal Sciences University, Bangladesh. Antimicrobial screening was determined by the disc diffusion method [13] against gram positive, gram negative bacteria and fungi.
|
2.3.2. Disc Diffusion Assay (DDA)
- Disc diffusion method is widely acceptable for the evaluation of antimicrobial activity. In this method, an antibiotic was diffused from a reliable source through the nutrient agar and a concentration gradient was created. Dried, sterilized filter paper discs (6mm diameter, HI-Media, China) containing the known amounts of test samples (400 μg/disc) were placed on nutrient agar medium consistently seeded with the test bacteria. As positive and negative control, standard antibiotic of ciprofloxacin (5μg/disc) and blank discs were used. For the maximum diffusion of the test materials to the surrounding media, these plates were reserved at low temperature (4°C) for 24 h. The plates were then incubated at 37°C for 24 h to allow optimum growth of the organisms. The test materials with antimicrobial property inhibited microbial growth in plates and thereby yielded a clear, distinct zone defined as zone of inhibition. The activity of the test sample was then determined by measuring the zone of inhibition expressed in millimeter [14].
2.4. Phytochemical Screening
- Testing of various chemical compounds within the extract, represent the preliminary phytochemical studies. Little amount of different fraction extracts of E. rubiginosum was subjected to preliminary quantitative phytochemical investigation for the detection of various phytochemicals [15, 16, 17, 18, 19].
2.5. Physicochemical Profile [20-25]
2.5.1. Ash Value
- Total ash, acid insoluble ash and water soluble ash were determined as reported in the Anonymous (1968) [20] and MHFW (1999) [21]. Briefly, total ash was determined using 2 g of the air-dried powdered sample. The total ash was boiled for 5 minutes with 25 ml of distilled water; the insoluble matter was collected on an ashless filter paper, washed with hot distilled water, and ignited for 15 minutes at a temperature not exceeding 450℃. The weight of the insoluble matter was subtracted from the weight of the total ash; the difference in weight represents the water-soluble ash. The percentage of the water-soluble ash was calculated with reference to the air-dried powdered plant sample.
2.5.2. Extractive Values and Moisture Content
- Extracts of the plant samples were prepared with different solvents for the study of extractive values. For present study alcohol and water were used as solvent for the study of extractives value [22,23,24,25].
3. Result
3.1. Brine Shrimp Lethality Bioassay
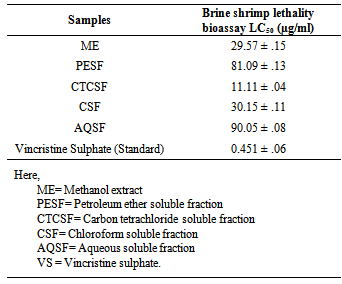
- In case of brine shrimp lethality bioassay, all the fractions of leaves of E. rubiginosum demonstrated significant cytotoxic potential against with LC50 values was ranging from 11.11µg/ml to 90.05 µg/ml as compared to 0.451 µg/ml for Vincristine sulphate (Table 2). The Carbon Tetra Chloride Soluble Fractions had shown highest cytotoxic activity with LC50 value of 11.11±0.04 µg/ml with compared to Vincristine sulphate.
|
3.2. Antimicrobial Screening
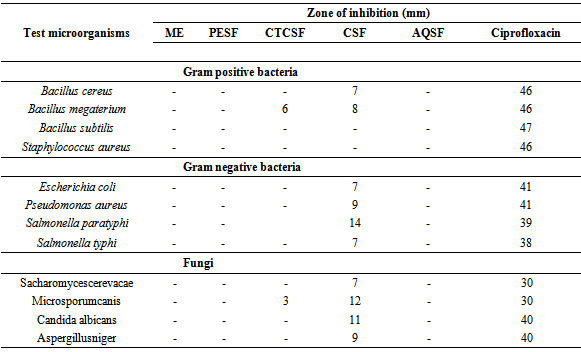
- The antimicrobial activity of different fractions extract of E. rubiginosum leaves was evaluated against four gram positive, gram negative bacteria and four fungi and the results were compared with standard (Ciprofloxacin). The test samples of E. rubiginosum revealed moderate antimicrobial activity with zone of inhibition ranging from 3.0 to 14.0 mm. The highest zone of inhibition (14.0 mm) was shown by the chloroform soluble fractions of leaves of the plant against Salmonella paratyphi. Interestingly only carbon tetra chloride soluble fraction and chloroform fraction had shown antimicrobial activity, where chloroform soluble fraction had shown activity to all pathogens except Bacillus subtilis, Staphylococcus aureus, on the other hand Carbon tetrachloride soluble fraction had shown activity to only Bacillus megaterium and Microsporumcanis (Table 3).
|
3.3. Phytochemical Screening
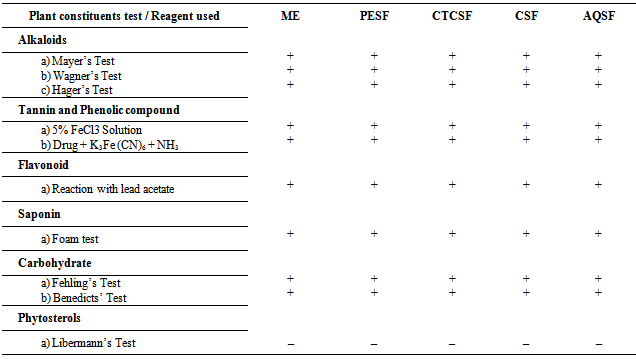
- In preliminary phytochemical screening, different test had been shown for six phytocompound where the different fraction extract of E. rubiginosum leaves demonstrated the presence of alkaloids, flavonoids, saponins, phenolic compound, carbohydrate and the absence of phytosterol (Table 4).
|
3.4. Physicochemical Profile
3.4.1. Ash Value
- Results of total ash value, acid insoluble ash and water soluble ash value have been shown in Table 5. Total ash value was found to be 8.50 %, acid soluble ash value 1.00% and water soluble ash value was found to be 0.50%.
|
3.4.2. Extractive Value and Moisture Content
- Results of the extractive value have been shown in Table 5. Alcohol soluble and water soluble extractive value were found to be 21.00% and 23.00% respectively. Moisture content was found to be 7.20%.
4. Discussion
- Recently, much attention has been directed towardplant extracts and biologically active compounds isolatedfrom popular plant species. The use of medicinal plantsplays a vital role in covering the basic health needs in developing countries and these plants may offer a newsource of cytotoxic, antibacterial, antifungal and antiviral agents with significant activity [26, 27]. Several studies have shown that brine shrimp bioassay has been an excellent method to screen the cytotoxic property of medicinal plants and for the isolation of a great variety of biologically active compounds [28]. Present investigation of the carbon tetrachloride soluble fraction of the plant had shown low LC50 value than any other fractions.On the basis of the result obtained in this present investigation, only the chloroform soluble fraction of E. rubiginosum leaves had moderate in vitro antimicrobial activity. This also revealed that the gram-positive bacteria were more resistant to the chloroform soluble fraction of E. rubiginosum than the gram-negative bacteria. Generally, gram-negative bacteria are more resistant to antibiotics than gram-positive bacteria [29,30]. The resistance is due to the differences in their cell wall composition. In gram-negative bacteria, the outer membrane acts as a great barrier to many environmental substances including antibiotics [31]. Presence of thick murine layer in the cell wall prevents the entry of the entry of the inhibitors [32]. The capability of the extracts to reveal antibacterial activity against the bacteria suggested the presence of hydrophilic and hydrophobic antibacterial compounds [33]. But the present study revealed a controversy report that gram-negative bacteria were more susceptible to the crude extracts than gram-positive bacteria. It may be due to the presence of broad spectrum of antimicrobial compounds in the leaves of E. rubiginosum.The phytochemical screening of methanol extracts and its fractions of E. rubiginosum leaves showed the presence of alkaloids, flavonoids, saponins, phenolic compound and carbohydrate. The biological activities of this plant may be due to the presence of those various groups of chemical compounds [34,35]. Presence of these types of important phytoconstituents reveals the usefulness of E. rubiginosum in various remedies. Presence of alkaloids, may be responsible for analgesic, antimicrobial, smooth muscle relaxant, anticencerous, and antioxidant activity [36]. Phenolic compounds and flavonoids, which can be mentioned as nature’s biological response modifiers, have shown anti-allergic, anti-inflammatory, antimicrobial, antidiabetic, antioxidative, antimutagenic, anticancer (cytotoxic) activities and saponin can be used as mild detergents and in intracellular histochemical staining [37]. The study exhibited the presence of flavonoids and polyphenolic compound as one of the major chemical constituents which are responsible for cytotoxic and antimicrobial activity [35]. Since the present study showed the presence of several bioactive secondary metabolites such as alkaloids, phenols, flavonoids, saponin and carbohydrate, these phytocompounds may be responsible for the defense mechanism against microorganisms and insects [38]. For the development of antimicrobial and cytotoxic agents, plants are important resources of potentially useful structures, because they are available, thus cost effective, minimal adverse effect, affordable, therefore in -vitro antimicrobial and cytotoxic assay is the preliminary step towards this goal. Physicochemical parameters of E. rubiginosum leaves prove that it retain good quantitative properties. Its dried form is likely to have a long shelf-life with reduced chance of microbial growth due to its relatively low moisture content of 7.20%. Total ash value of 8.50% shows low inorganic components in the herbal plant. Acid insoluble ash value of 1.00% specifies high digestibility when the plant is consumed. Water soluble ash value of 0.50% is indicative of negligible level of water-soluble minerals absorption from the plant when it is consumed. The alcohol-soluble extractive value of 21.00% and water-soluble extractive value of 23.00% confirms that both solvents would be good for extraction of this potential drug-plant.
5. Conclusions
- In light of the results of the present study, it can be summarized that the plant extract possesses low cytotoxic and moderate antimicrobial property with wide range of phytochemicals. The obtained results may provide a support to use of this plant in traditional medicine. Based on this, further chemical and pharmacological investigations to isolate, identify and to screen other potential bioactivities may be suggested.
ACKNOWLEDGMENTS
- The authors are grateful to BNH toidentify the plant, and CVASU to supply the microorganism. Authors are also thankful to Department of Pharmacy, Noakhali Science and Technology University, Bangladesh for providing the laboratory facilities.
Conflict of Interest Statement
- We declare that we have no conflict of interest.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML