-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Health Science
p-ISSN: 2166-5966 e-ISSN: 2166-5990
2014; 4(1): 11-17
doi:10.5923/j.health.20140401.03
Prescribed Daily Doses (PDDs) of Hypolipidaemic Agents in South Africa with Emphasis on HMG CoA Reductase Inhibitors
Ilse Truter
Drug Utilization Research Unit (DURU), Department of Pharmacy, Nelson Mandela Metropolitan University (NMMU), Port Elizabeth, 6031, South Africa
Correspondence to: Ilse Truter, Drug Utilization Research Unit (DURU), Department of Pharmacy, Nelson Mandela Metropolitan University (NMMU), Port Elizabeth, 6031, South Africa.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Dyslipidaemia is a major cardiovascular risk factor in the South African population. The primary aim of the study was to determine the prescribing patterns of hypolipidaemic agents on the South African market with specific emphasis on the Prescribed Daily Doses (PDDs) of HMG CoA reductase inhibitors (statins). A retrospective, cross-sectional pharmacoepidemiological study was conducted on 2011 claims data. All records for hypolipidaemic agents were extracted for analysis. A total of 4 805 patients (56.88% males) were prescribed 38 373 hypolipidaemic agents. The average age of patients was 56.07 (SD=13.32) years. Statins accounted for 93.85% of all prescriptions, followed by fibrates (3.61%). Simvastatin was the most frequently prescribed statin (accounting for 62.59% of all prescriptions), followed by atorvastatin (17.04%) and rosuvastatin (11.68%). The average PDDs were generally lower or in agreement with the Defined Daily Doses (DDDs), except for rosuvastatin. The average PDD of simvastatin was 23.70 mg (DDD=30 mg), pravastatin 25.35 mg (DDD=30 mg), lovastatin 26.31 mg (DDD=45 mg), atorvastatin 20.91 (DDD=20 mg) and fluvastatin 57.29 mg (DDD=60 mg). The average PDD of rosuvastatin was 15.02 mg and the DDD only 10 mg. The fibrates constituted 3.61% of prescribing. Only one cholesterol absorption inhibitor drug was prescribed (ezetimibe). Other hypolipidaemic agents prescribed accounted for only 0.89%. A variety of generic equivalents were available for the statins. Further studies are needed to investigate clinical parameters and diagnoses in relation to the PDDs.
Keywords: Prescribed Daily Doses (PDDs), Hypolipidaemic Agents, Statins
Cite this paper: Ilse Truter, Prescribed Daily Doses (PDDs) of Hypolipidaemic Agents in South Africa with Emphasis on HMG CoA Reductase Inhibitors, Journal of Health Science, Vol. 4 No. 1, 2014, pp. 11-17. doi: 10.5923/j.health.20140401.03.
Article Outline
1. Introduction
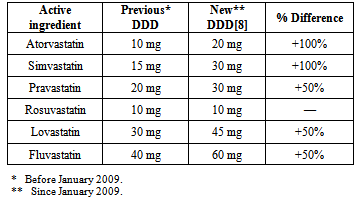
- Cardiovascular disease (CVD) is the leading cause of mortality worldwide[1]. Increased levels of low-density lipoprotein cholesterol (LDL-C) are an important modifiable risk factor[1]. The HMG CoA reductase inhibitors (or statins) are the most efficacious lipid-lowering drugs available. Statins lower LDL-C levels and have been reported to reduce the relative risk of coronary events by about 30% in both primary and secondary prevention[2, 3]. Statins are widely and increasingly used in most countries. Studies have, however, shown huge variations in use amongst countries and regions[3, 4].During the last decade clinical data on the benefits of statins in the prevention of cardiovascular disease have accumulated. High dose statins of all types are, however, associated with adverse effects such as myopathy and very rarely rhabdomyolysis. The risk is much higher in combination with certain medicines and in the elderly[5]. In February 2012, the United States Food and Drug Administration (FDA) had approved important safety label changes for the statins[6]. These changes were made to provide the public with more information for the safe and effective use of statins and are based on the FDA’s comprehensive review of the statins.The dosages in which statins are prescribed are therefore important. The WHO recommends the use of the defined daily dose (DDD) and prescribed daily dose (PDD) methodology in drug utilisation studies. The definition of the DDD is the assumed average maintenance dose per day for a drug used for its main indication in adults. DDDs are assigned by the WHO[7]. For instance, the DDD for atorvastain is 20 mg[8]. The prescribed daily dose (PDD) is defined as the average dose prescribed according to a representative sample of prescriptions[7]. The PDD can be determined from studies of prescriptions or medical or pharmacy records. The DDD is a unit of measurement and does not necessarily respond to the recommended or PDD, but the methodology is used internationally and allow for comparisons to be made.The DDDs for the statins remained unchanged from 1997. However, in January 2009, alterations were made to the DDD for five of the six statins in order to better reflect the current daily dosages[9]. Table 1 compares the previous DDD values (prior to January 2009) to the new DDD values (since January 2009) for each statin[8, 9]. The DDDs for atorvastatin and simvastatin have been doubled, whereas for pravastatin, lovastatin and fluvastatin the DDDs have been increased by 50%. There has been no alteration to the DDD for rosuvastatin.
|
2. Methodology
2.1. Research Design and Setting
- A retrospective, cross-sectional drug utilisation study was conducted on the database of a private medical insurance scheme administrator in South Africa. According to the South African Board of Healthcare Funders that represents 72 health insurers in South Africa, only 16% of the South African population is covered by medical insurance[11]. This equates to 3.5 million insured members and their 4.6 million dependents. The remainder of the South African population, that is 39.9 million people, is dependent on the government’s medical services for which detailed prescription data are not readily available. Electronically captured claims data for 2011 of a South African medical insurance scheme (medical aid) administrator were analysed in this study. The total database contained 2 298 312 records for medicine, medical devices and procedures and was representative of all provinces in South Africa.
2.2. Data and Statistical Analysis
- All records for hypolipidaemic agents were extracted for analysis. The Anatomical Therapeutic Chemical (ATC) Classification System[8], MIMS[12] and the South African Medicines Formulary[13] were used to identify medicines. Each medication record contained information on the age and gender of the patient, with a unique number to identify each patient, the date of the prescription, detailed information on the dispensed drug (name, package size, formulation, strength and quantity) and amount claimed and paid. Microsoft Access® and Excel® were used to analyse the data. Basic descriptive and inferential statistics were calculated. One Euro (€1.00) was equal to R9.81 (South African Rand), one US Dollar ($1.00) was equal to R6.76 and one British Pound (£1.00) was equal to R10.85 at the time of the study (30 June 2011).
2.3. Ethical Approval
- Ethical approval to conduct studies on prescription databases has been obtained from the Research Ethics Committee (Human) of the Nelson Mandela Metropolitan University (ethics clearance number: H08-HEA-PHA-005).
2.4. Limitations of the Study
- Limitations of the study were that no clinical information or diagnoses were available in the database, and that only data of patients served by the private health care sector in South Africa were included in the study. Patients served by the government or state’s health care system (the public health care sector) were therefore not included in the study.
3. Results and Discussion
3.1. Age and Gender Distribution of Patients
- A total of 4 805 patients (56.88% males) were prescribed 38 373 hypolipidaemic agents during 2011. The percentage age and gender distribution of patients is given in Figure 1. The average age of patients was 56.07 (SD=13.32) years. The average age of female patients was 57.32 (SD=13.92) years and of male patients 55.12 (SD=12.77) years. Differences between females and males in the different age groups were observed (2=62.718; d.f.=7; p<0.0001). There were more males in the age groups between 40 and 59 years, and more females in the age groups older than 60 years.
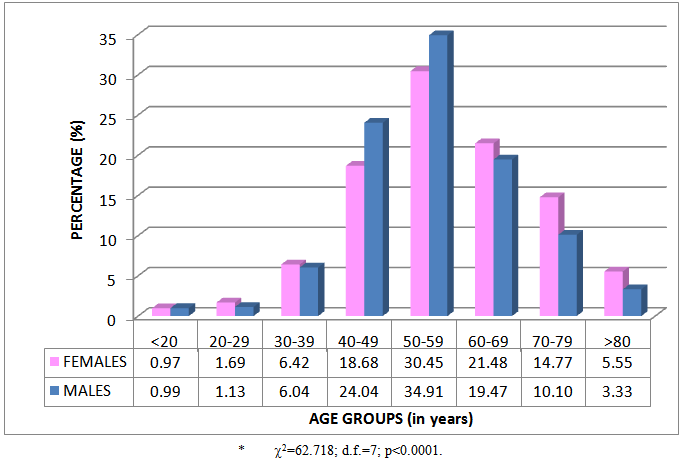 | Figure 1. Percentage age and gender distribution of patients (n=4 805)* |
3.2. Prescribing Frequency of Hypolipidaemic Agents
- The percentage prescribing frequency of hypolipidaemic products according to age and gender groups is given in Figure 2. Patients were prescribed on average 7.99 (SD=4.85) hypolipidaemic products during the year. Female patients were prescribed on average 7.92 (SD=4.80) products and male patients 8.03 (4.89) products. Prescribing differences between females and males were observed (2=554.86; d.f.=7; p<0.0001). Proportionately more products were prescribed to male patients between 30 and 59 years of age, and after the age of 60 years proportionately more female patients were prescribed hypolipidaemic products.
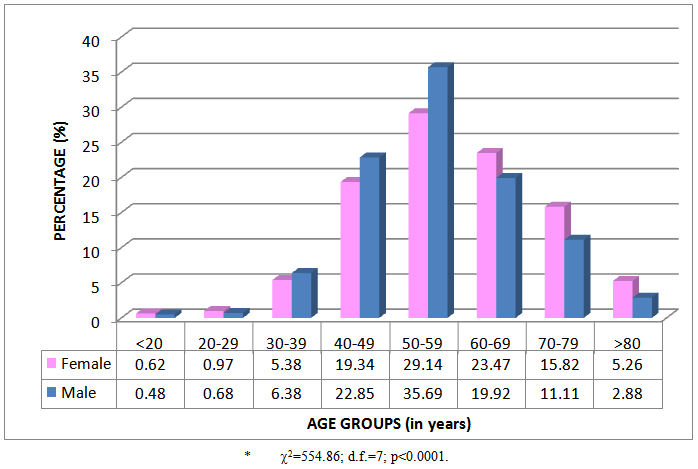 | Figure 2. Percentage prescribing frequency of hypolipidaemic agents according to age and gender groups (n=38 373)* |
3.3. Prescribing Frequency of Hypolipidaemic Classes
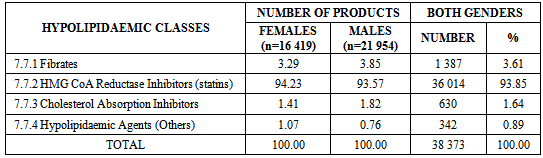
- Statins accounted for 93.85% of all prescriptions, followed by fibrates (3.61%) (see Table 2).
|
3.4. Active Ingredients Prescribed
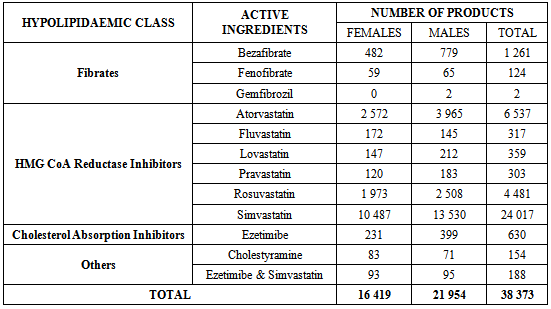
- Simvastatin was the most frequently prescribed statin (accounting for 62.59% of all prescriptions), followed by atorvastatin (17.04%) and rosuvastatin (11.68%) (see Table 3). The fibrates constituted 3.61% of prescribing, with most prescriptions for bezafibrate. Only one cholesterol absorption inhibitor drug was prescribed (ezetimibe) accounting for 1.64% of prescriptions. Other hypolipidaemic agents prescribed accounted for only 0.89% and consisted of the combination of ezetimibe and simvastatin, and cholestyramine.
|
3.5. DDDs and Average PDDs of the Statins
- The average PDDs of the statins were generally lower or in agreement with their respective Defined Daily Doses (DDDs), except for rosuvastatin whose average PDD was higher (see Figure 3). The average PDD of simvastatin was 23.70 mg (DDD=30 mg), pravastatin 25.35 mg (DDD=30 mg), lovastatin 26.31 mg (DDD=45 mg), atorvastatin 20.91 (DDD=20 mg) and fluvastatin 57.29 mg (DDD=60 mg). The average PDD of rosuvastatin was 15.02 mg and the DDD only 10 mg. Although the average PDD of rosuvastatin was higher than the DDD, it was still within the acceptable dosage range. The dosage range for rosuvastatin is 10 mg to 40 mg[13].
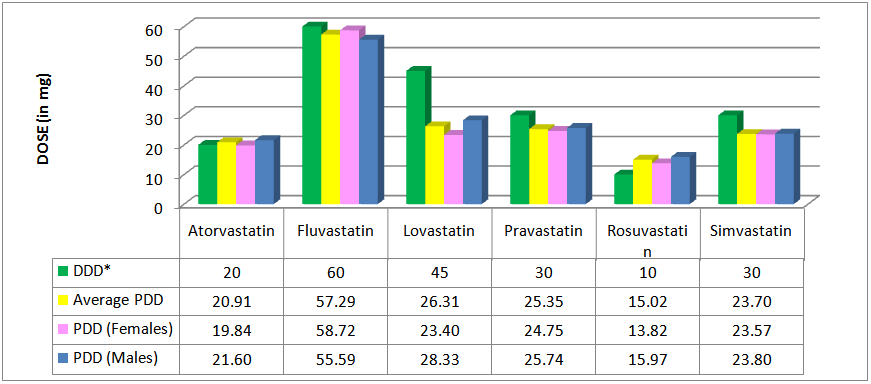 | Figure 3. DDDs and average PDDs of the statins |
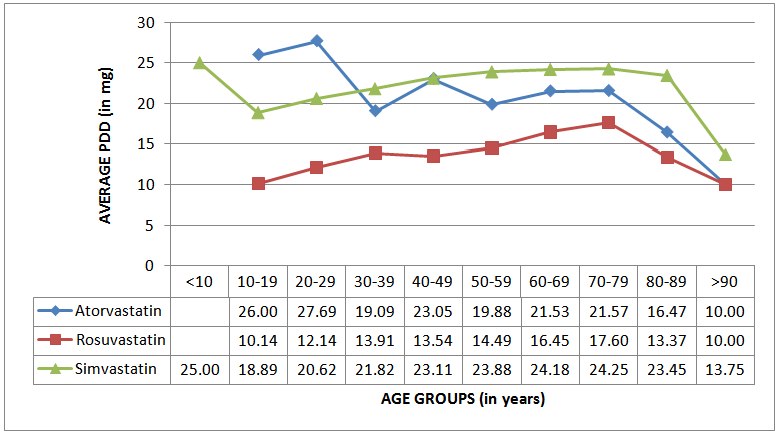 | Figure 4. Variability according to age groups of the average PDDs of the three most often prescribed statins |
4. Conclusions
- The HMG CoA reductase inhibitors were the most often prescribed class of hypolipidaemic agents in this study, accounting for 93.85% of all prescriptions. Simvastatin was the most often prescribed active ingredient, accounting for 62.59% of all hypolipidaemic prescriptions. The average PDDs were generally lower or in agreement with the established DDDs, except for rosuvastatin. Differences were observed in the pattern of lipid-lowering drug prescriptions for females and males. Male users were on average younger than female users.It is important to relate PDDs to the diagnosis on which the dosage is based. This was a limitation of this study since diagnoses and clinical data were not available. Usually when there is a substantial discrepancy between the PDD and the DDD, it is important to investigate this further when evaluating and interpreting drug utilisation figures, particularly in terms of morbidity.More detailed studies investigating the relationship between patients’ cholesterol levels and PDDs are therefore needed to determine the structure of the relationship between the severity of the raised lipid level and the PDD. In addition, there are a variety of generic equivalents available for the statins on the South African market. Further studies can be conducted to investigate the cost implications of generic prescribing.
ACKNOWLEDGEMENTS
- The medical insurance scheme administrator for providing the data for the study.This work is based upon research supported by the National Research Foundation (NRF). Any opinion, findings and conclusions or recommendations expressed in this paper are those of the author and therefore the NRF do not accept any liability in regard thereto.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML