-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Health Sciences
p-ISSN: 2166-5966 e-ISSN: 2166-5990
2012; 2(5): 43-48
doi: 10.5923/j.health.20120205.01
Hypoglycemic and Biochemical Effects of Matricaria Chamomilla Leave Extract in Streptozotocin-Induced Diabetic Rats
Najla O. A. , Olfat A. K. , Kholoud S. R. , Enas N. D. , I Hanan S. A.
Department of Biochemistry, Faculty of Girls Science, King Abdulaziz University Jeddah, Saudi Arabia
Correspondence to: Olfat A. K. , Department of Biochemistry, Faculty of Girls Science, King Abdulaziz University Jeddah, Saudi Arabia.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The aim of this study was to evaluate the hypoglycemic and biochemical effects of the water extract of leaves of Matricaria chamomilla in Streptozotocin (STZ; 45 mg./kg, i.p.) -induced diabetic rats. The water extract was administered to non-diabetic and STZ-induced diabetic albino rats. Parameters including blood glucose, insulin, kidney function , liver function and lipid profile were tested using standard kits and methods after administration of the extract. Histological changes in the liver and kidney of the animal were also examined. The results obtained revealed that the 200 mg kg-1 body weight Matricaria chamomilla once daily for 21 days reduced the elevated Fasted Blood Glucose (FBG) by 62.2 % (p < 0.001). The values of urea, creatinine, Uric acid, AST, ALT, ALP, TC, TG and LDL-c were also reduced after Matricaria chamomilla treatment in diabetic rats. The histological changes in liver showed microvesicular steatosis after induction of diabetes. While, Diabetic kidney sections showed coagulative necrosis of the tubules in the corticomedullary junction. The results demonstrate that the water extract of Matricaria chamomilla possesses a strong hypoglycemic effect in STZ-induced diabetic rats.
Keywords: Matricaria Chamomilla, Hypoglycemic Effect, Liver, Streptozotocin-Induced Diabetic Rats, Blood Glucose
Cite this paper: Najla O. A. , Olfat A. K. , Kholoud S. R. , Enas N. D. , I Hanan S. A. , "Hypoglycemic and Biochemical Effects of Matricaria Chamomilla Leave Extract in Streptozotocin-Induced Diabetic Rats", Journal of Health Sciences, Vol. 2 No. 5, 2012, pp. 43-48. doi: 10.5923/j.health.20120205.01.
Article Outline
1. Introduction
- Diabetes is a chronic disease characterized by hyperglycemia and many macrovascular complications resulting from defects in insulin secretion (1). The macrovascular complications of diabetes are associated with oxidative stress induced by hyperglycemia (2). There is possibility of hyperlipidemia and liver damage in the later stages of diabetes due to disorders in lipid metabolism and increased gluconeogenesis and ketogenesis (3). The side effects of antidiabetic drugs has led to use several species of medicinal plants with hypoglycemic properties (4). The hypoglycemic properties of these plants are reported to be due to their higher contents of flavonoides and different bioactive compounds.Chamomile (Matricaria chamomilla L.), known in ancient Egypt and Rome, is one of the common medicinal plants used in the world. The main active components of M. chamomilla L. are bisabolol, bisabololoxide, bisabolonoxide, and chamazulene. It contains 0.75% of a volatile oil that is blue in colour (5). Matricaria recutita is well known for its pharmaceutical properties such as anti-inflammatory, immunomodulatory activity, arcaricadal property,anti-cancer activity and antipruritic effect (6). Some reports suggest that flavonoids play an important role in inflammatory processes by inhibition of different enzymes which are activated during inflammation (7), such as activation of antioxidative enzymes (8).With regard to these properties, this study is designed to evaluate the hypoglycemic and biochemical effects of Matricaria chamomilla water extract to be used in the traditional management of diabetes.
2. Materials and Methods
- This study was carried out between December, 2011 to June, 2012 at the Department of Biochemistry, King Abdulaziz University, Jeddah, Saudi Arabia. Leaves of Chamomile were obtained from the local herbal market of Kingdom Saudi Arabia. Voucher specimens from plant material were deposited at the Herbal Museum, Department of Pharmacology, Faculty of Science, King Abdulaziz University of Medical Sciences for identification.
2.1. Preparation of the Plant Sample
- The fresh leaves of plant material (5 g) were soaked in 50 ml of boiled water, after 1 h stirring, at room temperature. The supernatant was decanted and the residue was macerated two more days with distilled water. The pooled supernatants were combined and filtered.
2.2. Animals and Treatment
- Adult male albino rats were selected for the study. They were of the same age (2 months) and weight (150 to 200 g). The animals were housed in acrylic cages in standard conditions of temperature prior to the experiments for 1 week in order to adapt to the laboratory condition, fed with commercial diet and water ad libitum. The principles of laboratory animal care were followed throughout the duration of experiment and instruction given by King Abdulaziz University ethical committee was followed regarding experimental treatments.
2.3. Experimental Design and Treatment Schedule
- The experiment was carried out on 4 groups of five rats in each group to study the effect of plant water extract on STZ -induced diabetes as follows: Group 1: Healthy control rats, received distilled water. Group 2: Diabetic control rats. A freshly prepared solution of Streptozotocin or STZ (45 mg/kg body weight in 0.1 M citrate buffer, pH 4.5) was injected intraperitonially into overnight fasted rats. STZ injected animals exhibited hyperglycemia within 48 to 36 h (9). The rats having fasting blood glucose (FBG) values of 250 mg/dl or above were considered for the study. Group 3: Normal rats administered water extract of Chamomile, orally at a dose of 200 mg/kg body weight (dosage determined earlier, (8), and Group 4: Diabetic treated rats received 1 ml water extract of Chamomile for 21 days after 36 h STZ injection. The treatment with Chamomile 200 mg/kg/day (10) was given daily for a period of 3 weeks using gastric cannula (11). No detectable irritation, restlessness or adverse effect (respiratory distress, abnormal locomotion or catalepsy) was observed in any animals after the extract administration. Starting from the 1st day (3rd day of STZ-injection) of extract administration to diabetic rats, FBG (blood glucose) level was measured in every 7th day using glucometer (12). On the 21th day of extract administration, all the animals were anesthetized (Nesdonal 50 mg/kg, i.p.), blood samples were obtained from hearts of overnight fasted rats by using micro-capillary technique and allowed to clot for 20 min in laboratory temperature and then centrifuged at 10000 rpm for 10 min for serum separation. The kidney and liver were excised immediately and thoroughly washed in ice – cold saline. The serum and tissues were collected and used for biochemical and histological experiments.
2.4. Biochemical Parameters
- Serum insulin concentrations were determined by radioimmunoassay kit (Awareness Technologies, USA) with a beta metric counter. The kit included human insulin as standard and 125I labeled human insulin antibody, which cross-reacts similarly with rat insulin. Blood urea nitrogen (BUN), serum creatinine (Scr) and uric acid were determined as markers of kidney function by using the commercially available kits from Siemens Health Care Diagnostics according to their manufacturers.All the enzymes were determined within 24 h of sample collection. The liver biomarkers such as Aspartate aminotransferase (AST), Alanine aminotransferase (ALT), Alkaline phosphatase (ALP), Total protein (TP), and Albumin were assayed in serum spectrophotometrically by standard automated techniques according to the procedures described By the manufacturers. Serum lipid profile including serum levels of triglycerides (TG), total cholesterol (TC), low density lipoprotein cholesterol (LDL-c), very low density lipoprotein and high density lipoprotein cholesterol (HDL-c) were measured biochemically by using the commercially available kits from Siemens Health Care Diagnostics according to their manufacturers.Histological study: After blood sampling, the kidneys and livers were removed and fixed in 10% neutral buffered formalin for 24 h, washing was done in tap water then serial dilutions of alcohol (methyl, ethyl and absolute ethyl) were used for dehydration. Specimens were cleared in xylene and embedded in paraffin at 56 degree in hot air oven for twenty four hours. Paraffin bees wax tissue blocks were prepared for sectioning at 4 microns by slidge microtome. The obtained tissue sections were collected on glass slides, deparaffinized and stained by hematoxylin and eosin stains for histopathological examination through the electric light microscope.Statistical Analysis: The results were expressed as mean ± standard deviation (SD). The data were subjected to one-way analyses of variance (ANOVA) and student’s t-tests using the Statistical Analysis System (SPSS 15.0) program. For all analyses, p values ≤ 0.05 were considered significant.
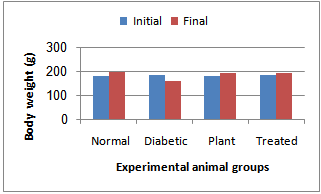 | Figure 1. Body weight changes in normal and experimental animals in each group at the initial and final days after treatment |
3. Results
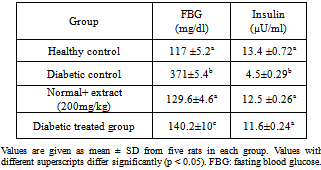
- Fig. 1 shows the body weight changes in the normal and experimental animals in each group. The mean body weight of the diabetic rats was decreased as compared to control rats. The body weight loss of diabetic rats treated with Chamomile was lower as compared to non-treated diabetic rats.Glucose levels were found to be significantly increased, while insulin levels were significantly decreased after STZ administration. There after administration of chamomile extract showed decrease in the levels of glucose and increase in blood insulin when compared to the diabetic control group (Table 1).
|
3.1. Effect of Chamomile on Serum Kidney Functions
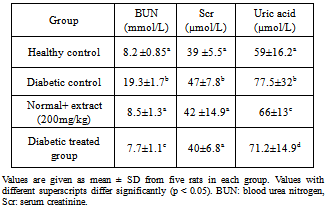
- Diabetes significantly increased serum urea, creatinine and uric acid in comparison with the control group. Treatment of diabetic animals with 100 mg/kg/day chamomile extracts significantly inhibited increase of urea, creatinine in comparison with the untreated diabetic animals (p< 0.05). These treatments can maintain the levels of urea and creatinine at the same levels compared to that of the control group (Table 2).
|
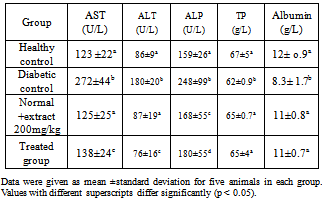
3.2. Effect of Chamomile on Serum Liver Functions
- Table 3 shows the levels of serum liver enzymes in normal and experimental rats. Administration of the water extract restored the levels of the serum enzymes to near normal levels.
|
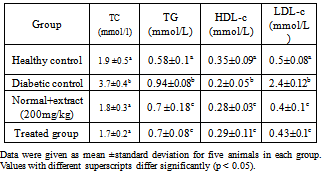
3.3. Effect of Chamomile on Lipid Profile
- The effects of chamomile on serum lipids of normal and experimental rats is summarized in Table 4. A marked increase in the frequency of cholesterol, triacylglycerol and LDL-c were observed in diabetic control rats. Treatment with chamomile extract significantly reduced the lipid levels.
|
3.4. Liver Histology
- Group I (control rats) showed that the portal tracts were composed of portal vein radical, and bile duct radical. The central veins appeared normal. In diabetic rats, the hepatocytes showed the global microvasicular steatosis, dilated bile duct with inflammatory cell infiltration in the portal area. In the nondiabetic rats treated with chamomile the hepatocytes, portal tracts and central veins appeared normal. In diabetic rats treated with chamomile, the hepatocytes shows mild inflammation, central veins appear normal. No steatosis was observed (Plate 1).
3.5. Kidney Histology
- Diabetic kidney section showed coagulative necrosis of the tubules in the corticomedullary junction with cystic dialation and hemorrhage. Treatment kidney shows periglomerular inflammatory cells infiltration Plate 2).
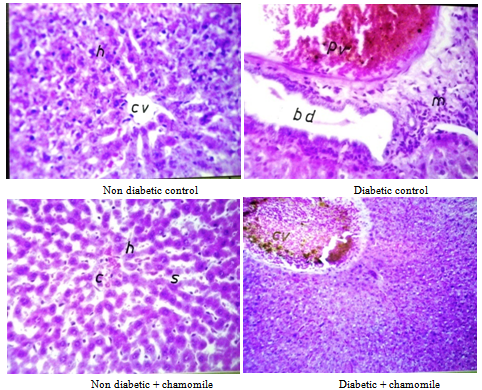 | Plate 1. Liver sections of the rat |
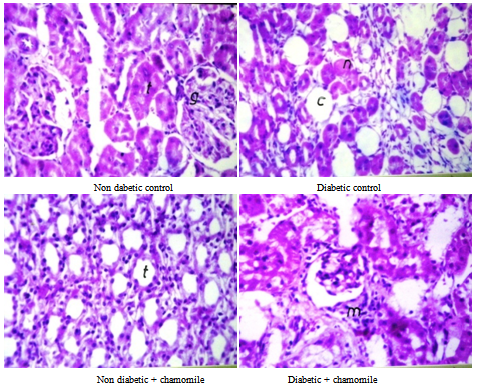 | Plate 2. Kidney sections of the rat |
4. Discussion
- The use of natural products for medicinal application in their natural available form is the general trend now (13). Plants have been effectively prescribed for the treatment of many diseases including diabetes mellitus. The demand for herbal remedies is growing as various types of hypoglycemic agents, although controlling blood sugar may produce a number of side effects (14). The hypoglycemic activity of some plant extracts has been evaluated and confirmed in animals and in human beings (Sharma et al., 2010). Several methods have been used for induction of diabetes mellitus in animals. STZ is commonly used for induction of experimental diabetes (15).In the present study administration of STZ to the rats caused increase in blood glucose level and decrease in level of blood insulin (p < 0.001). Treating the diabetic animals with the water extract of chamomile caused a significant reduction (p< 0.001) in blood glucose level and increase in the level of blood insulin. These changes may be due to the depletion of the pancreatic β-cells inducted by STZ causing hyperglycemia and decrease in insulin production and release (16).The oral administration of chamomile water extract exhibited strong antihyperglycemic effect and improved the state of hypoinsulinemia in diabetic rats. That is because chamomile has a favourable effect to inhibit the histopathological changes of the pancreas in STZ-induced diabetes (6). A number of other plants have also been reported to have antihyperglycemic and insulin-release stimulatory effect (10, 17, and 18).In this study it was found that there was body weight loss of diabetic rats when compared to the control group. This loss is due to increased muscular wasting on loss of tissue protein (19). The ability of chamomile to recover body weight loss can be due to its antihyperglycemic effect and improvement in insulin secretion. In a study of Saravanan and Leelavinothan (20) on diabetic rats treated with S. cumini extract, they reported that the significant gain in body weight after treatment compared to the diabetic control may be due to its protective effect in controlling muscle wasting i.e. reversal gluconeogenesis and glycogenolysis.The results of this study revealed significant increase (p< 0.001) of serum levels of urea (BUN), creatinine (Scr) and uric acid of diabetic rats compared to control healthy rats. After treatment with chamomile extract, Scr and BUN decreased to the normal level while uric acid level remained high. The decrease in serum levels of urea and creatinine in chamomile treated diabetic rats may be due to the antioxidant activity of chamomile. Haidara et al., (21) reported inhibition of urea and creatinine in diabetic animal treated by vitamin E. Antioxidant therapy to the diabetic patients is one of the most important treatment strategies for prevention and inhibition of diabetic nephropathy progression (10). Nirmala et al., (17) reported that no histological changes in the kidney sections were noticed in STZ-induced rats and rats fed with B. rubra.The present study revealed significance increase (p< 0.05) of serum AST, ALT and ALP while total protein and albumin are significantly decreased in diabetic rats compared to the controls and returned to normal levels after treatment with chamomile. Assay of liver enzymes AST, ALT and ALP indicated a decrease in their activities in chamomile treated diabetic rats compared to the diabetic untreated group and the control group.The increase in the activities of the liver enzymes indicated that diabetes may be induced due to liver dysfunction leading to leakage of these enzymes from the hepatocytes into the blood stream (22). Assay of these enzymes are important in the diagnosis of liver damage caused by drug toxicity or harmful chemicals (23). The increase in serum levels of these enzymes is considered as marker of liver dysfunction (24). The hepatic marker enzymes that are highly increased in STZ-induced diabetic rats, give an indication on the hepatotoxic effect of STZ. The decrease of the liver enzymes in chamomile diabetic rats indicates the hepatoprotective effect of chamomile. In this study serum cholesterol (TC), triacylglycerol (TG) and LDL-c are significantly (p< 0.05) increased in diabetic group compared to the control one while HDL-c is decreased. After treatment with chamomile extract cholesterol returned to normal level while the changes of serum levels of TG, HDL-c and LDL-c were significantly differ from the control healthy group. The increase in serum levels of cholesterol and TG may be due to the uninhibited actions of lipolytic hormones on the fat depots (25) or the increase in the metabolism of free fatty acids from the peripheral fat depots (26). Estakhr and Javdam, (27) reported that the cause of depletion of glucose, TC and TG in hyperglycemia condition after using M. recutita extract is the inhibition of the enzyme glycogen phosphorylase catalyzes glycogenolysis. This action inhibits glucagon which according to feedback inhibition favours the production of insulin (28).In agreement with our results, Sharma et al. (29) reported that altered lipid and lipoprotein profile, i.e. increase in TC, TG and LDL-c with fall in HDL-c was reversed towards normal level after oral administration of M. rubra extract in STZ-induced diabetic rats.
5. Conclusions
- It can be concluded that chamomile water extract may be helpful in reducing the complications of hyperlipidemia and hypercholestremia which are actively raised in STZ-diabetes rats.
ACKNOWLEDGMENTS
- This work was supported by Research Grant from University of King Abdulaziz, Jeddah.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML