-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Health Science
p-ISSN: 2166-5966 e-ISSN: 2166-5990
2012; 2(2): 8-13
doi: 10.5923/j.health.20120202.03
Effects of Momordica Charantia on Streptozotocin-Induced Diabetes in Rats: Role of Insulin, Oxidative Stress and Nitric Oxide
Nagy M A 1, Bastawy M A 1, Abdel-Hamid N M 2
1Chemistry Department, Faculty of Science, Beni-Suef University, Egypt
2Biochemistry Department, Faculty of Pharmacy, Minia University, Egypt
Correspondence to: Abdel-Hamid N M , Biochemistry Department, Faculty of Pharmacy, Minia University, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Oxidative stress associated with insulin –dependent diabetes mellitus is a risk for vascular disorders and diabetic nephropathy. Bitter melon(BME) is a widely used as antioxidant and vasorelaxant. This study to evaluate hypoglycemic, hypolipedimic and antioxidant effects of aqueous Momordica charantia extract against streptozotocin (STZ) induced diabetes in male rats and explore possible effect of BME on insulin and nitric oxide release. Method: This study was carried on 30 male Sprague-Dawley rats weighing 80-90 g, 60 days old, classified into 3 groups, control, diabetic and treated diabetic group. STZ was given in a dose of 30 mg/kg for 2 consecutive days intraperitoneally (IP). Result: STZ produced a significant increase in serum glucose , triglyceride (TG), total cholesterol (TC), low density lipoprotein cholesterol (LDL-C), urea and creatinine (Cr), significant increase in renal reduced glutathione (GSH), nitric oxide (NO), significant decrease in insulin levels, myeloperoxidase (MPO) and erythrocyte glutathione–S-transferase (EGST)) activities. Oral BME (400 mg/kg/d/8 weeks) significantly ameliorated these effects. Conclusion: Treatment with BME improved DM and its related late consequences. Furthermore, it has antioxidant and vasodilator effects. Commonly, BME is a way to surmount the diabetic state and it has vasodilator effects. It may be a promising adjuvant to anti diabetic therapy.
Keywords: Bitter Melon, Diabetes Mellitus, Lipid Profile, Nephropathy, Oxidative Stress
Article Outline
1. Introduction
- The most common three types of diabetes mellitus (DM) are :type I (juvenile diabetes), type II (late onset diabetes) and gestational diabetes. All are manifested by elevated blood glucose level. In type I Diabetes, pancreatic β cells fail to produce sufficient insulin. Type II diabetes is the major type of diabetes, accounting for approximately 90–95% among all diabetic cases.Diabetic nephropathy is a leading cause of end-stage renal disease and endothelial dysfunction is a central pathophysiologic denominator for all cardiovascular complications of diabetes including nephropathy[24]. Blood glucose fails to enter cells, so, its blood is increased. Persistent elevated blood glucose can damage nerves and blood vessels, leading to complications such as heart disease, strokes, kidney dysfunction, blindness, polyneuropathy, gum infections and foot amputation[22]. Insulin injections, glucose-lowering drugs and lifestyle changes, exercise, weight and diet control, are recommended for diabetic management. Nitric monoxide (NO) maintains effective vascular function and stimulates blood flow within the whole body. It is produced in vascular endothelial cells. When released into blood, it triggers vascular smooth muscles relaxation and activates peripheral blood flow. Certain herbs were reported to lower blood glucose, although their efficiency is still debatable. Firstly, each herb contains a lot of components, few of which may be therapeutically effective. Secondly, different parts of an herb have different ingredient profiles[44]. Moreover, extraction methods may yield different active ingredients. Finally, herbal formulae containing multiple herbs may have synergistic effects[10]. Momordica charantia (MC, family Cucurbitaceae), commonly known as karela, bitter gourd, balsam pear, or bitter melon (BM) is a tropical and subtropical vine, widely grown as edible fruit among the most bitter of all vegetables[6].The seeds, fruit, leaves, and root of the plant have been used in traditional medicine for microbial infections, sluggish digestion and intestinal gas, menstrual stimulation, wound healing, inflammation, fever reduction, hypertension, and as a laxative and emetic. Clinical conditions for which MC fruit extracts are currently being used in treatment of diabetes, dyslipidemia, microbial infections, and potentially as a cytotoxic agent for certain types of cancer[31]. Although they have not been definitively determined, research indicates the primary constituents responsible for the hypoglycemic properties of Momordica are charantin, insulin-like peptide (plant (p)-insulin), cucurbutanoids, momordicin, and oleanolic acids. P-insulin is structurally and pharmacologically similar to bovine insulin and is composed of two polypeptide chains held together by disulfide bonds[28].The present work was conducted to evaluate the possible hypoglycemic, hypolipidemic and antioxidant properties of BME in streptozotocin (STZ)- induced type I diabetes mellitus (DM).
2. Material and Methods
2.1. Materials
- Experimental animalsThirty male albino rats (Sprague Dawley strain) weighing 150-200 gm, around 120 days old were used in this study. They were purchased from the National Research Center, Giza, Egypt. All animals were housed in stainless steel cages containing barriers between the rats. They were kept in environmentally controlled room with temperature of 24±5℃, illumination (12 h light/12 h dark cycle), a relative humidity of 60±4%. Water and rodent chow (composed of fat 5%, carbohydrates 65%, proteins 20.3% fiber 5%, salt mixture 3.7% , g/kg) were available ad libitum throughout the period of the investigation[25]. The chow was purchased from El-Gomhoria Company, Cairo, Egypt. They were housed for two weeks for accommodation. Our work was carried out in accordance with the guidelines of Minia University for animal use. These animals were used for induction of Diabetes mellitus.
2.2. Plant Material Preparation of BME
- The whole ripe fruits (purchased from local suppliers) were homogenized in cold phosphate-buffered saline (PBS) (8.00 g/liter), filtered through cheese cloth then centrifuged at 16,300 x g () for 20 min. The resulting supernatant was precipitated to 50% saturated ammonium sulfate, and the resulting pellet was taken up in PBS and dialyzed against PBS overnight to remove residual ammonium sulfate. The suspension had the least amount of water, being a soft extract. All procedures were conducted at 4℃ unless otherwise stated. This crude aqueous extract was stored at -70℃ in 50 ml aliquots and used as the source of crude bitter melon preparation, it is stable for up to 1 year at -70℃.The extract was prepared at the Materia Medica Department at College of Science, Beni Suef University.
2.3. Experimental Design and Animal Grouping
- Type I DM was induced in 20 rats by a double-IP injection of STZ (Sigma) as 30 mg / kg for two consecutive days with a total of 60 mg / kg / rat. STZ was dissolved in citrate buffer (0.05 M, pH 4.5). Rats with blood glucose over 160 mg/dl were considered as diabetic[37]. These DM animals were divided into 2 groups, one served as untreated DM group, the other as treated DM group, which was given BME as 400 mg/kg daily, one week apart from last STZ second dose for 8 consecutive weeks[36]. Control rats were injected with same volume of vehicle (citrate buffer). At the end of the experiment, all groups were bled by vein puncture, fasting blood samples were centrifuged, sera were kept at -80℃ right the time of analysis, meanwhile, kidney and brain were collected, blotted between 2 filter papers, frozen directly into liquid Nitrogen, then kept at -80℃ till tissue biochemical investigations.
2.4. Methods
- Biochemical investigations in blood and tissueIn erythrocyte, GST activity was assayed[21], using reduced glutathione (GSH) as substrate. The assay mixture of 2 ml contained 0.5 Mm l-chloro-2,4-dinitrobenzene (CDNB), 10M GSH and 100 mM Potassium phosphate buffer, pH 6.5.CDNB was dissolved in 2% ethanol and added to the phosphate buffer before use. Exactly 0.40 ml phosphate buffer, 0.04 ml CDNB solution and 1.36 ml distilled water were introduced in a test tube and incubated for 10 min at 37℃. 0.1 ml GSH was then added and shaken vigorously, 0.1 ml hemolysate of each of the test samples was added to each of the test tubes and then transferred into a 2 ml cuvette. Absorbance was read at 340 nm. The change in optical density (O.D) was monitored for 10 min at 30 sec intervals. GST activity was calculated as described by Habig et al. (1974) using values of hemolysate hemoglobin concentration.Serum glucose was estimated according to the method of Trinder[39], using Stanbio Laboratory Kits, USA. Serum insulin was assayed in the Radioactive Isotopes Unit, Central Department of Scientific Analysis and Test, National Research Center (Dokki, Giza) using radioimmunoassay kits (Diagnostic Products Corporation, Los Angeles, USA) according to the method of Marschner et al.,[27]. Serum total cholesterol levels was enzymatically determined with a colorimetric[5] and triglyceride by enzymatic method of Grossman et al.,[19]. HDL-cholesterol and LDL-cholesterol were estimated using precipitant[18] and Friedewald formula[17]. Serum creatinine was determined utilizing Jaffe’s reaction[38] and urea was determined[9].For determination of tissue biochemical parameters, rats were sacrificed by decapitation and incisions were immediately done for separation of the kidney and brain. The isolated kidney was quickly weighted and dissected into pieces, homogenized in volumes of ice cold de-ionized water to yield 20% W/V homogenate using ice cold Teflon homogenizer (Potter Elvehjem type). Renal GSH content was measured[33]. Also, renal NO was determined in the homogenate as nitrite[16]. For assessment of renal MPO (EC1.11.1.7) activity, the supernatant was discarded and the pellet was used for the assay as described previously[32].Statistical analysisStatistical analysis was carried out using Graph Pad Instat software (version 3, ISS-Rome, Italy). Groups of data were compared with ANOVA, followed by Tukey-Kramer (TK) multiple comparisons post-test. Values of P < 0.05 were regarded as significant. Data were expressed as mean ± standard error (SEM).
3. Results
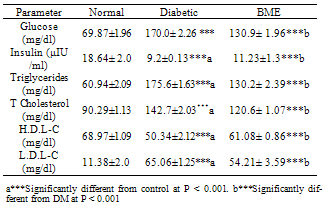
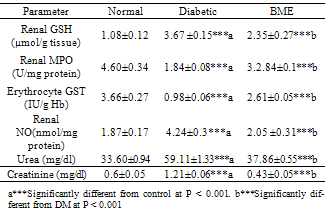
- Serum glucose levels were significantly increased, but insulin levels were decreased in STZ diabetic rats compared to control, and treatment with BME significantly reduced glucose but non significantly elevated insulin in relation to diabetic rats. Activity of erythrocyte GST was significantly decreased in STZ group compared to control, while treatment with BME significantly ameliorated these changes. Both blood creatinine and urea levels were significantly increased in STZ group compared to control, while treatment with BME significantly ameliorated these changes. Both triglycerides, total cholesterol and LDL-C levels were significantly increased, while HDL-C was significantly decreased by induction of diabetes, these figures were significantly ameliorated after BME treatment (Table 1). Renal GSH and NO contents were significantly increased, with a significant decrease of MPO activity, in the STZ group compared to control, treatment with BME significantly ameliorated these changes (Table 2):
|
|
4. Discussion
- BM is a popular vegetable that is widely grown in tropical areas. Documentation of its pharmacological properties back to the 16th century. Although BM was found to possess antiviral, antibacterial, anti-HIV, anticancer, and immunomodulatory properties, attention has been focused on its blood glucose–lowering effect. Such an effect was demonstrated in STZ-induced diabetic, [4] and diet-induced obese (DIO) rats[20].STZ is a widely used inducer of DM in experimental animals. It causes selective destruction of pancreatic islet cells of Langerhans[41]. The possible mechanisms for β-cell destruction by this chemical was through generation of some types of oxygen free radicals and alteration of endogenous scavengers of these reactive species, fragmentation of DNA, increase in the activity of poly–ADP ribose synthase (an enzyme known to deplete nicotinamide adenine dinucleotide in β-cells), inhibition of ATP synthesis and islet mitochondrial respiratory enzymes[30].The present study revealed that intra-peritoneal (IP) injection of STZ lead to hypoinsulinemia and hyperglycemia as an evidence of diabetogenesis . This is in agreement with that reported elsewhere[7] who noticed a significant reduction in the number of α and β cells of islets of Langerhans in STZ –treated guinea pigs.In present study, adult male albino rats were used , since male in generally are in general more susceptible to diabetes than females[2] and adults were used since young ones have a higher resistance to the diabetogenic effect of STZ.STZ diabetic rats showed a highly significant increase in serum glucose level compared to normal rats. These results run parallel with that of Abdel–Moneim et al.[3]. STZ selectively destructs β-cells of the islets of Langerhans in the pancreas resulting in inhibition of insulin synthesis and elevation of blood glucose level due to, firstly, a reduced entry of glucose to peripheral tissues, muscle and adipose tissue, secondly, increased glycogen breakdown and finally, increased gluconeogenesis and hepatic glucose production[34].BME shows significant decrease in serum glucose with significant increase in level of insulin in accordance to Shetty et al.[33] and Dans et al.[13]. MCE contains a lectin that has insulin-like activity, due to its affinity to insulin receptors. It lowers blood glucose levels by enhancing peripheral cellular uptake of glucose, increasing glucose utilization by the liver via improvement of glucose oxidation through activating glucose-6-phosphate dehydrogenase with decrease of gluconeogenesis via inhibition of two key enzymes, glucose-6-phosphatase and fructose-1,6 - bisphosphatase[23].On other hand, Oleanolic acid glycosides (compounds from BM) improved glucose tolerance in diabetics by preventing sugar from being absorbed into intestines,[40]. The other possible mechanism is that BME increases the number of insulin producing beta cells in the pancreas of diabetic animals, promotes insulin release and potentiates its effect. However, it should be noted that stimulating insulin release is probably less desirable than improving insulin sensitivity[37]. BME significantly decreased LDL cholesterol and TG in accordance with the observations of Yadav et al.[43] and Yin et al.[44]. The defatted part of the seeds (rich in fibers and steroid saponins) is responsible for the hypocholesterolemic action of seed components, interaction with bile salts in the digestive tract, as well as, the enhancement of cholesterol secretion into the bile [10].BME showed significant decrease in renal GSH and significant increase in activity of MPO and erythrocyte GST, these actions were noticed previously[10,15,45]. Chan LL, [10] reported that MCE is a good source of phenolic compounds which possess potent antioxidant effect that may enhance detoxification mechanisms including glutathione- dependent conjugation reactions resulting in additional protection against free radicals. Possibly, these compounds are catabolized by GSH. MCE depicted non significant effect on NE content among diabetic rats STZ diabetic rats showed significant increase in renal NO content as compared to normal rats in accordance with Napoli and Ignarro[29]. In glomerulonephritis, there is intraglomerular activation of inducible nitric oxide synthase (iNOS) leading to production of nitric oxide (NO). This can result in supraphysiologic amounts of NO and cause oxidative injury that forms reactive nitrogen species, which may further impair mitochondrial respiration and can even lead to opening of the mitochondrial transition pore and cell death. Diabetes have been associated with altered reactive oxygen substance (ROS) generation, which can alter the delicate regulatory balance of NO in the mitochondria[14]. These observations shed further light on alterations of NO, not only in the course of vascular complications but in the pathogenesis of diabetes itself. MCE showed significant decrease in renal level of NO in accordance with Lii et al[26].BM is rich in special amino acid called citrulline which can increase the amount of nitric monoxide and vitamin C which can eliminate active oxygen quickly to keep nitric monoxide longer in the blood vessels when arginine availability is limited. Once, citrulline is taken into the body, it is changed back to citrulline within the liver by the enzyme called Peptidyl Arginine Deiminase, Type IV (PADI4). This process is repeated within the liver, and NO is produced as a by-product in this process Wu G, Bazer FW[12,42].The protective mechanism of NO in the kidney is impaired during the development of diabetes, probably via the actions of ROS and a decrease in tetrahydrobiopterin, thus contributing to the pathogenesis of diabetic nephropathy. Restoration of this protective NO mechanism can be achieved by simultaneous stimulation of NO synthesis and prevention of the effects of ROS through the use of L-arginine and a combination of vitamins C as diet supplement that forms the components of BM extract in this study[11].STZ diabetic rats showed highly significant increase in level of serum urea and creatinine as compared to normal ones that is in agreement with[25]. oxidative stress have close association with diabetic renal damage. BME was reported to induce significant decrease in serum level of urea and creatinine[1] that may be attributed to a power of BME to eliminate the toxins from the body through the urine, in addition to having a diuretic effect.
5. Conclusions
- Treatment with BME improved associated metabolic consequences to Type I DM, showing hypoglycemic, insulin sensitization, antioxidant and hypolipidemic actions. The results suggest BME as a beneficial adjuvant for the treatment of type I diabetes mellitus and possibly as a protector against long term nephropathy. These correlations are summarized in Fig 1:
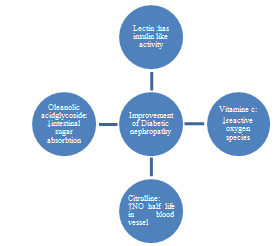 | Figure 1. Shows the synergistic effects between different components of BME |
Conflict of interest statement
- There are no conflicts of interest.
ACKNOWLEDGEMENTS
- We appreciate the assistance and advice of Prof Bastawy M., Vice Dean of College of Science and Dr. Kamal Adel Amin, , for their kind co-operation.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML