-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Health Science
p-ISSN: 2166-5966 e-ISSN: 2166-5990
2012; 2(2): 1-4
doi: 10.5923/j.health.20120202.01
Antimicrobial Activities of Telfairia occidentalis (fluted pumpkins) Leaf Extract against Selected Intestinal Pathogens
Oyewole O. A. , Abalaka M. E.
Department of Microbiology, Federal University of Technology, PMB 65, Minna, Niger State, Nigeria
Correspondence to: Oyewole O. A. , Department of Microbiology, Federal University of Technology, PMB 65, Minna, Niger State, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The antibacterial assay of the leaf of Telfairia occidentalis (Fluted pumpkins) on Salmonella typhi, Escherichia coli and Streptococcus faecalis was determined using the agar diffusion technique to investigate its potential use as antibacterial agent. The Minimum Inhibitory Concentration (MIC), Minimum Bactericidal Concentration (MBC) and phytochemical components of the leaf were studied. The phytochemical screening of the extract indicated the presence of glycosides, saponins, flavonoids, phenolics and steroids. The extract showed a higher antibacterial activity against E. coli (20 ±0.58mm at 500mgmg.ml), S. faecalis (6 ± 1.10mm at 5.0mg) and S. typhi (11±0.70mm at 50mg/ml). The MIC were 0,5mg/ml, 5.0mg/ml and 500mg/ml for E. coli, S. typhi and S. faecalis respectively. The MBC for E. coli and S. typhi were 0.5mg/ml and 5.0mg/ml respectively while S. faecalis was resistant to the extract. The result of the study suggests that leaf extract of T. occidentalis can be used for the treatment of infections by the test organisms.
Keywords: Telfairia Occidentalis, Leaf Extracts, Intestinal Pathogens, Agar Diffusion
1. Introduction
- According to the World Health Organization (WHO) a medicinal plant is any plant which in one or more of its organ contains substances that can be used for the synthesis of useful drugs (World Health Organization, WHO, 1977). Medicinal plants contain biologically active chemical substances such as saponins, tannins, essential oils, flavonoids, alkaloids and other chemical compounds (Sofowora, 1996) which have curative properties. These complex chemical substances of different compositions are found as secondary plant metabolites in one or more of these plants (Kayode and Kayode 2011).Telfairia occidentalis commonly called fluted pumpkin occurs in the forest zone of West and Central Africa, most frequently in Benin, Nigeria and Cameroon (Kayode and Kayode 2011). It is a popular vegetable all over Nigeria. It has been suggested that it originated in south-east Nigeria and was distributed by the Igbos, who have cultivated this crop since time immemorial. It is, however, equally possible that fluted pumpkin was originally wild throughout its current range, but that wild plants have been harvested to local extinction and are now replaced by cultivated forms (Badifu and Ogunsina, 1991, Kayode and Kayode 2011).The medicinal activities of Telfairia occidentalis has been reported by many investigators. In Nigeria, the herbal preparation of the plant has been employed in the treatment of anaemia, chronic fatigue and diabetes (Alada, 2000; Dina et al., 2006; Kayode and Kayode 2011). The leaves contain essential oils, vitamins; root contains cucubitacine, sesquiterpene, lactones (Iwu, 1983). The young leaves sliced and mixed with coconut water and salt are stored in a bottle and used for the treatment of convulsion in ethno medicine (Gbile, 1986). The leaf extract is useful in the management of cholesterolemia, liver problems and impaired defense immune systems (Eseyin et al., 2005). Telfairia occidentalis is popularly used in soup and folk medicine preparation in the management of various diseases such as diabetics, anaemia and gastrointestinal disorder (Oboh et al., 2006). A study has shown that the ethanol root extract of T. occidentalis possess antiplasmodial potential (Okokon et al., 2007) and inhibitory effects on some enterobacteriacae Odoemena and Onyeneke (1998) while Oluwole et al. (2003) reported Telfairia occidentalis anti-inflammatory activities (Kayode and Kayode 2011). The aims and objectives of this research are to identify the phytochemical components of the leaf of Telfairia occidentalis, to determine the antimicrobial activities of the leaf extract of Telfairia occidentalis, to determine the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of Telfairia occidentalis on S. typhi, E. coli and Strept. faecalis, to compare the antibacterial activities of the leaf extract of Telfairia occidentalis-with antibiotics commonly used to treat infections of the test organisms.
2. Materials and Methods
- Collection and preparation of SamplesFresh leaves of T. occidentalis were purchased in Bosso market in Minna, Niger State, Nigeria and brought to the microbiology laboratory of Federal University of Technology, Minna. The leaves were air-dried at room temperature over a period of one week. The dried leaves were pulverized to dry powder using a wooden mortar. Fifty grams (50mg) of dried leaves were extracted in a succession using 250ml of 75% ethanol in a separate conical flask for 72 hours with regular agitation. The extract was evaporated to dryness using steam bath and stored in sterile universal bottle as described by Sliver et al. (1997).Pure culture of the organisms were obtained from stock culture of microbiology laboratory, Federal University of Technology, MinnaExtraction of the extractOne hundred grams (100g) of the blended leaf was soaked in 500ml of 75% ethanol in a flask It was shaken daily for 72hours at regular intervals, after which it was filtered suing Whatman’s (No II) filter paper. The filtrate was evaporated to dryness using steam water bath at 100oC. The extract was then stored at 4oC in a refrigerator.Phytochemical screening of the extractsPhytochemical screening of the extracts was carried out according to methods described by Odebiyi and Sofowora (1978) and Trease and Evans (1989). The components analyzed for were alkaloids, tannins, phenolics, glycosides, saponins, flavonoids, steroids. Phylobatanins and triterpenes.Determination of Minimum Inhibitory Concentration (MIC)The MIC of the plant extract was determined by serially diluting extract from 101 to 1010. One millilitre (1ml) of each of the dilutions representing a known concentration of the extract was introduced into 9ml of sterile nutrient broth in a test tube. The mixture was then inoculated with 0.1ml of the test organisms previously standardized at 106. It was then incubated at 37oC for 24 hours. The least concentration of the plant extract in the test tube with no turbidity was taken as the MIC (Hugo and Russsel 1983).Determination of Minimum Bactericidal Concentration (MBC)The plant extract was serially diluted from 10 to 1010. One millilitre (1ml) of each of the dilutions representing a known .concentration of the extract was introduced into 9ml of sterile nutrient broth in test tubes. The mixture was then inoculated with 0.1ml culture of the test organisms previously standardized to 106. It was then incubated at 37oC for 24hours. The least concentration of plant extract in the test tube with no turbidity was taken as the MIC. Subsequently, tubes that indicated no turbidity was plated out on nutrient agar plates and absence of growth after incubation for 24hours confirms the MBC as described by Hugo and Russell (1983).
3. Results
- Phytochemical Screening of extractTable 1 shows the phytochemical components of extract of Telfairia occidentalis. The result indicated the presence of saponins, alkaloids, tannins, phenolics, and absence of glycosides, steroids, phylobatanins, triterpenes
|
|
|
|
4. Discussion
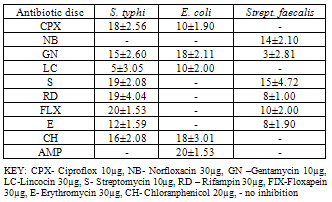
- The preliminary phytochemical analysis showed that the leaf of T. occidentalis contains tannins, saponins, alkaloids and flavonoids. These compounds have been found to inhibit bacterial growth and are capable of protecting certain plant against bacterial infections (Clark, 1981; Gonzales and Mather, 1982). At concentrations of 500mg/ml, 50mg/ml and 5.0mg/ml, E. coli had the highest zones of inhibitions (20±0.58mm) whereas, at concentrations of 50mg/ml, Strept. faecalis was tolerant to the extract of T. ocicidentalis and at 5.0mg/ml S. typhi was also tolerant (Table 2). This indicated that E.coli was the most susceptible to the leaf extract of T. occidentalis at the various concentrations used. At concentration of 500mg/ml, all the test microorganisms had higher zones of inhibition when compared to the commonly used antibiotic (Table 4 and Table 5).The minimum inhibition concentration of the test organisms were 0.5mg/ml for E. coli, 5.0mg/ml for S.typhi and 500mg/ml for Strept. faecalis (Table 3). This is similar to the finding of Oboh et al. (2006) who reported inhibitory effects of ethanolic extract of T. occidentalis on , Pseudomonas aeruginosa and Proteus sp. but no inhibitory effect on Salmonella typhi. The minimum bactericidal concentration (MBC) of 0.5mg/ml and 50mg/ml appeared to be bactericidal on S. typhi and E. coli respectively but Strept. faecalis had no bactericidal activity on extracts of T. occidentalis at 500mg/ml, 50mg/ml and 5.0mg/ml. This could be due to its ability to develop resistance to the extracts of T. occidentalis at concentrations considered and also because of the presence of peptodoglycan cell wall component.
5. Conclusions
- The results of the study indicates that the ethanolic extract of T. occidentalis has an antibacterial activity on S. typhi, E. coli and Strept. faecalis. E.coli was most susceptible (MIC and MBC values of 0.5mg/ml) followed by S.typhi (MIC and MBC values of 5.0mgml) while S. faecalis was the least susceptible.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML