-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Journal of Health Science
p-ISSN: 2166-5966 e-ISSN: 2166-5990
2012; 2(1): 1-4
doi: 10.5923/j.health.20120201.01
The Reno-Protective Effects of Coconut Water on the Kidneys of Diabetic Wistar Rats
Eze K. Nwangwa
Department of Physiology, College of Health Science, Delta State University, P.M.B. 001 Abraka, Nigeria
Correspondence to: Eze K. Nwangwa , Department of Physiology, College of Health Science, Delta State University, P.M.B. 001 Abraka, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
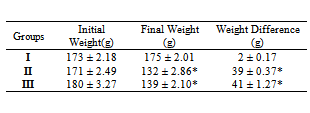
Diabetes Mellitus is one of the leading causes of end stage renal failure. In this present study the remedial effect of coconut water on alloxan-induced diabetes mellitus on the total body weight, kidney function and kidney cyto-architecture were studied. A total of eighteen (18) wistar rats were randomly divided into three experimental groups (n=6). Group I (Control) was fed on rat chow and water ad libitum and Group II and III were made diabetic by single intra peritoneal dose of 150mg/kg body weight of alloxan monohydrate. Group II were fed rat chow and water ad libitum and Group III fed a rat chow and coconut water ad libitum for 30 days. The result showed a statistical significant (P ˂ 0.05) reduction in weight, a significant increase in the renal function parameters (urea, creatinine, potassium, sodium and bicarbonate) due to diabetes and a significant reduction in these parameters following the administration of coconut water. The renal cyto-architecture also showed a protective/regenerative effect following treatment with coconut water. This finding may be beneficial in preventing kidney damage in diabetic patients.
Keywords: Renal Histology, Renal Function Test, Coconut (Cocos Nucifera) Water
Cite this paper: Eze K. Nwangwa , "The Reno-Protective Effects of Coconut Water on the Kidneys of Diabetic Wistar Rats", Journal of Health Science, Vol. 2 No. 1, 2012, pp. 1-4. doi: 10.5923/j.health.20120201.01.
Article Outline
1. Introduction
- According to the American Diabetes Association (ADA) (2004), diabetes is a metabolic disease caused by excess glucose in the blood and is characterized by hyperglycemia resulting from defects in insulin secretion, action or both. In diabetes the level of blood glucose is persistently raised above normal range [80 – 100]mg/dl, (Peter et al, 1996). Uncontrolled diabetes mellitus causes varied histopathological changes in different organs (Harold, 1978; Thomas, 1999) and incidences of diabetic nephropathy has been on the increase (Adewole et al., 2006; Carrington and Litchfield, 1999; Clements and Bell, 1982).Coconut water, an isotonic beverage with the same level of electrolyte balance as that of human blood, was regularly used to give emergency plasma transfusion to wounded soldiers in the pacific war (Campbell – Falck et al, 2000). It has less sodium (25mg) than energy drinks (200mg) and sport drinks (41mg), it is very high in chloride at 118mg compare to 38mg of sport drinks (George et al., 2004). According to Bruce (2000), coconut water contains 95.3 % water, 0.005% nitrogen, 0.56% Phosphoric acid, 0.25% Potassium, 0.69% Calcium Oxide, 0.59% Magnesium Oxide, 0.5% Iron, 0.8% reducing sugar and a total sugar of 2.08%.Coconut water is more nutritious than whole milk for itsless fat and no cholesterol, more healthy than orange juice for it is much lower calories and better than processed baby milk as it contain lauric acid which is present in human mother’s milk (George et al., 2004). Coconut water is used as therapeutic means of fighting against viruses (Ranti et al, 1965), it also replenishes the body’s fluid after exercise, lowers cholesterol, re-hydrates the body and can carry nutrients and oxygen to cells (Thomas et al, 2000).According to Campbel-falck (2002), coconut water contains organic compound posing healthy growth promoting properties that have been enough to help treat kidney and urethral stones. Nigerian researchers of the faculty of basic medical sciences, university of Illorin examined the blood sugar lowering effect of coconut water, extracted from Picralima nitida seed. In conclusion it was shown that coconut water extracted of P. nitida seed have a significant hypoglycemic (lowering) effects in alloxan induced diabetes (Salihu et al, 2009).The aim of the present study therefore, is to investigate the effect of administration of coconut water on the renal function and histology in alloxan-induced diabetic wistar rats.
2. Materials and Methods
2.1. Animals and Animal Handling
- Eighteen (18) adult rats of the Wister strain weighing between 170g – 180g of both sexes were used and these rats were purchased from the Animal Centre Benin City, Edo State, Nigeria. They were housed individually, in special clear-sided cages of same sizes at the Animal Centre of the Faculty of Basic Medical Sciences, Delta State University, Abraka for 14 days acclimatization and duration of the experiment. The rats were kept at room temperature and humidity and were given rats chow and water ad libitum. The coconut water was gotten from coconut fruits bought from Ubulu-uku market, Ubulu-uku in Delta State. The coconut fruit was authenticated in the department of Botany, Delta State University, Abraka, Nigeria.
2.2. Induction of Diabetes
- Diabetes was induced by a single intraperitoneal administration of freshly prepared solution of alloxan monohydrate (150mg/kg) (Pari and Mahesulari, 1999), and 5 days later, one touch glucometer (Lifescan Johnson and Johnson, Carlifonia) was used to confirm diabetes using blood samples collected from the tails of the rats at blood glucose level ≥ 250mg/dl.
2.3. Experimental Design
- The rats were randomly divided into three experimental groups (n=6). Group I (Control) was non diabetic fed on normal rat chow and water ad libitum. Groups II and III were diabetic fed on normal rat chow plus water ad libitum and normal rat chow plus coconut water ad libitum respectively. All administration of water, coconut water and rat chow was between the hour of 8am and 6pm which lasted for the duration of 30 days.
2.4. Tissue Processing/Photomicrography
- The rats were sacrificed after thirty days and their kidney harvested, observed microscopically fixed in formal saline and then prepared for tissue photomicrograph.
2.5. Statistical Analysis
- All data were expressed as mean ± SD (Standard Deviation).The results were analyzed by one-way analysis of variance (ANOVA), followed by comparison between test and control groups using Student’s t-test. Differences between groups were considered significant at p < 0.05.
3. Results
|
|
3.1. Microscopic Examination of the Kidney
- Plate 1 (Group 1) – normal control) shows the intact Bowman’s capsule. The glomerulus (A) contains cells with tuft of capillaries. The distal convoluted tubules show normal outline. The proximal convoluted tubules show single cuboidal epithelial cells with normal cellular structure (B) with normal interstitial space (C).
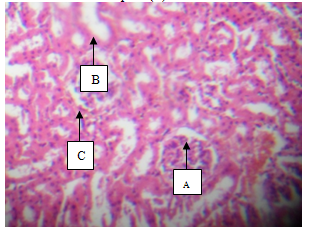 | Plate 1. showing Glomerulus (A), Tubules (B) and interstitial space (C) (H & E, x100) |
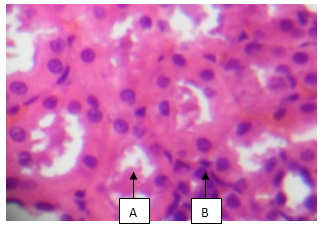 | Plate 2. Showing Tubular Protein cast (A), and chronic inflammation with fibrosis (B) (H & E x10) |
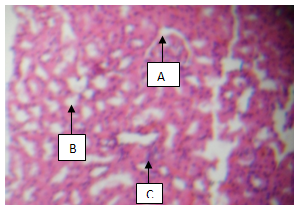 | Plate 3. showing Glomerulus (A), Tubules (B) and Interstitial Space with mild inflammation (C) (H & E, x100) |
4. Discussion
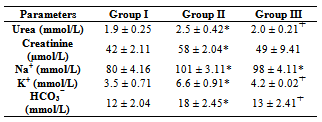
- The result from this present study shows that diabetes mellitus causes a significant (p<0.05) reduction in the body weight of rats as shown in Group II compared with Group I (Control) rats and concomitant administration of coconut water did not cause any significant difference in the weight. This finding is contrary to the report of Loperito and Rajamohan (2003) who reported that coconut water causes a further decrease in the weight of diabetic rats. Weight loss is a well documented clinical feature of diabetes mellitus which may be due to degeneration of the adipocytes and muscle tissues to produce energy and over conversion of glycogen to glucose (Reno and Lekerd, 1999; Zink and Chaffin, 1998)There is also a significant (P < 0.05) increase in the levels of urea (2.5 ± 0.42 mmol/L), Creatinine (5.8 ± 2.04 µmol/L), sodium (101 ± 3.11 mmol/L), Potassium (6.6 ± 0.91 mmol/L) and Bicarbonate (18 ± 2.45mmol/L) in diabetic non treated rats (Group II) compared with the Control (Group I) and treatment with coconut water (Group III) caused a significant (P < 0.05) reduction in urea (2.0 ± 0.21 mmol/L), potassium (4.2 ± 0.52 mmol/L ) and bicarbonate (13 ± 2.41 mmol/L) compared with Group II. This further explains the destructive effects of diabetes mellitus on the kidney with the resultant increased incidence in diabetic nephropathy (Collier et al, 2006).The histology of the kidney of the Control (Group I) shows glomerulus with normal capillaries with the Distal Convoluted and Proximal Tubules showing normal cellular structures compared with that of diabetic rats (Group II) which shows some degeneration of the glomerulus with presence of Tubular cast and signs of chronic inflammation. Peter et al (2006) previously reported a damaging effect of diabetes in the glomerulus thereby affecting Glomerular filtration rate (GFR). However, these findings were significantly but partially reversed by the concomitant administration of coconut water (Group III).
5. Conclusions
- This present study shows that diabetes mellitus has a degenerative and destructive effect on the kidneys which can be partially but significantly reversed by concomitant administration of coconut water.
ACKNOWLEDGEMENTS
- The researcher express his gratitude to Iyawa Onyeka for the preliminary work in this subject matter and to the staff of Chemical pathology and Histopathology Department of University of Benin Teaching Hospital for their technical support.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML