O. M. Alile 1, S. Oranusi 2, O. O Adetola 3, J. O. Airen 1
1Department Of Physics, University Of Benin, Benin City, Nigeria
2Department Of Biological Sciences, Covenant University, Ota, Nigeria
3Department Of Physics, Covenant University, Ota, Nigeria
Correspondence to: O. M. Alile , Department Of Physics, University Of Benin, Benin City, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Abstract
Geoelectrical imaging of the subsurface to investigate groundwater contaminants in a sedimentary environment like Ota, South-western Nigeria was carried out. The 2 – Dimensional electrical resistivity survey method for the subsurface imaging was done by engaging the Wenner array configuration using PASI Resistivity Meter in the surface measurement. The vertical electrical sounding (VES) was also done using the Schlumberger array configuration to determine the depth to the aquifer layer. The DIPRO software and the WIN-Resist application software were used for the interpretation of the 2 – D resistivity data and VES data respectively. Both Hydrochemical and Microbial analyses were done to test for the presence of heavy metals and the estimation of Total Aerobic Bacteria (TAB). From the VES results the depth to the aquifer lies around 20.1m (60.33ft.) of resistivity of 34.1 Ώ-m and the 2–D results showed the resistivities as 28.0 Ώ-m and 47.0 Ώ-m respectively showing that the study area has groundwater potential. For the hydrochemical analysis on heavy metals, the result lies within World Health Organization (WHO) standard for drinking water. But for the microbial analysis, total aerobic plate count of 2.3× for drinking water is high and above WHO standard specification for drinking water.
Keywords:
Geoelectrical, Imaging, Hydrochemical, Microbial, Analysis
Cite this paper:
O. M. Alile , S. Oranusi , O. O Adetola , J. O. Airen , "Subsurface Geophysical Investigation and Physiochemical/Microbial Analysis of Groundwater Contaminant in Ota, Southwestern Nigeria", Geosciences, Vol. 2 No. 6, 2012, pp. 179-184. doi: 10.5923/j.geo.20120206.05.
1. Introduction
The subsurface geophysical imaging engaging the use of 2 – Dimensional electrical resistivity and vertical electrical sounding methods was carried out to investigate the groundwater potential. The hydrochemical and microbial analysis of the groundwater in the study area was also done. Groundwater occurs in the saturated zone of the earth crust. It is derived from precipitations that infiltrate the soil and percolate down into cracks and inter granular void spaces in either the bed rock or unconsolidated sediments (1).The quality and quantity of available water have implication on the health status of a community. Over 50,000 people die daily due to water borne diseases (2), (3) and mortality in children under five years from water related diseases annually is estimated to be about 4 million in developing countries and 2.3 billion people worldwide have mortality and morbidity associated with water related ailment (4), (5), ( 6).Groundwater quality has become a major source of concern in recent years, particularly through Man’s activities which have further declined the quality. The consequences of surface runoff and groundwater percolation can severally affect the water quality in floodplain areas (7), (8), (9) and (10). In Nigeria, groundwater is exploited through the means of bore holes and wells of various depths.Groundwater contamination occurs when man-made products such as gasoline, oil, road salts and chemicals get into the groundwater and cause it to become unsafe and unfit for human use. Some of the major sources of these products, called contaminants, are storage tanks, septic systems, hazardous waste sites, landfills, and the widespread use of road salts, fertilizers, pesticides and other chemicals. The major sources of groundwater contamination include the following aspects; Geophysical aspect, Chemical aspect, Microbial aspect and Man-Made aspect. While many of the chemicals and substances that are regulated may be naturally occurring (calcium, sodium, iron, manganese, etc.) the concentration if often the key in determining what is a natural component of water, and what is a contaminant (11).Groundwater pollution is the addition of chemical, physical or biological substances of heat which causes determination in the natural quality, generally through the action of man or animal or any kind of activity (12). Ground water is being widely contaminated and its quality is determined by a large of variables which may be considered in terms of bacteriological content, physical characteristics such as temperature, turbidity, colour, taste, odour and chemical properties.Water pollution affects plants and organisms living in water bodies and in almost all cases the effect in damaging either to individual species and populations, but also to the natural biological communities. Pollution of water is realised when contaminants gain access directly or indirectly into water bodies without sufficient treatment to remove harmful substance.The study area is located between 6°40'35"N and 3°10'2"E, Ota, Ogun State, South-western Nigeria. Ogun State is bounded in the West by the Benin Republic, in the South by Lagos State and the Atlantic Ocean, in the East by Ondo State and in the North by Oyo State. The study was carried out around two different large and heavily pressured septic tank and the environment in which the septic tanks are situated.
2. Methodology
The method adopted in this study is the engagement of the 2 – Dimensional electrical resistivity survey method and vertical electrical sounding (VES), using the PASI resistivity meter (terrameter) in the geophysical measurement. The interpretations of the data were done by using the DIPRO software package on the imaging and the WINRESIST application software for the VES data. Each water sample was collected in a chemically clean plastic bottle with double seal from borehole in the study area. Distilled water was first used to rinse the bottle to avoid contamination. The samples were collected in the afternoon and toward evening after the borehole have been put to use. The concentration of heavy metals was determined using the Atomic Absorption Spectrophotometer (AAS). Instrumental calibration was carried out prior to metal determination by using standard solutions of metal ion prepared from salts. Commercial analar grade 1000ppm stock solutions of Ni2+, Cu2+, Pb2+, Fe2+ were diluted in 25cm3 standard flask and made up to the mark with deionized water to obtain working standard solutions of 2.0ppm, 3.0ppm and 4.0ppm of each metal ion. The sample water was digested using concentrated nitric acid HNO3 and concentration of Lead (Pb), Nickel (Ni), Iron (Fe), and Copper (Cu) measures with S series atomic absorption spectrophotometer (AAS). The essence of the digestion before analysis was to reduce organic matter interference and convert metal to a form that can be analyzed by AAS.Estimation of total aerobic bacteria (TAB) was done by plate count technique. Hundred folds serial dilution of 1ml of water sample was carried out two times in a set of test tubes each containing 9ml sterile distilled water. Then 0.1ml of each dilution was plated out respectively in duplicates employing the use of nutrient agar medium (sterile) kept in molten form, spread plate method was adopted. The culture plates were incubated, invertedly and aerobically at 35℃ for 48 hours. The enumeration is for only aerobes and facultative heterotrophic bacteria. The plates were observed for growth and selected for count after the expiration of the incubation period. The culture plate on which the number of colonies was less than 300 (and its duplicate) for each sample was selected (the significant count for diluted sample in 300 colonies). The averaged count was multiplied by the dilution factor at that dilution and expressed as the colony forming unit (CFU) per millilitre of the original water sample. This is a viable count.The most probable number (MPN) of coliform presumptive test was carried out on each of the test samples by inoculating three portions in each of three dilution in geometric series employing the use of single and double strengths lactose broth. For inoculating three portions in each of three dilutions in geometric series, a set of three test tubes, each containing 10ml double strength broth (sterile), two sets of three test tubes, each containing 5ml sterile single strength broth were required. Ten millilitres of a sample was inoculated into each tube of double strength, 1ml of the sample was inoculated into each of the first set of three tubes of single strength and 0.1ml of the same sample was inoculated into each of the other set of three tubes of the single strength. The culture tubes were carefully agitated to mix the inoculation with the broth medium. They were incubated at 35℃ for 48 hours and each tube observed for growth and gas production. The combined numbers of positive tubes in each set, arranged in order of least diluted to the most diluted tubes was read out from the appropriate standard MPN table to obtain the estimated number of coliform cells present in 100ml of the original water sample.Tubes showing gas and/or acid (MPN) tubes) are subcultured in eosin methylene blue (EMB) agar incubated at 35℃ for 24 hours and examined for typical colonies of Escherichia coli (greenish metallic sheen) of a typical colony are seen; the completed test is carried out.Several typical colonies of Escherichia coli from the EMB plate are sub cultured into brilliant green broth fermentation tubes and peptone water. The fermentation tube was incubated at 37℃, and another one at 44℃ both for 48 hours. The peptone water was incubated at 35℃ for 24 hours. Gas in the fermentation tube and a gram negative short rod, indole positive is evidence of Escherichia coli. Escherichia coli produces acid and gas from the broth at 44℃ and at lower temperature in motile and indole positive. So positive tubes in both broth tubes incubated at 37℃ and 44℃ in an indication of faecal coli that occur normally in the human and animal intestine and it is (natural to assume that their presence indicates recent contamination with faeces. As such, pathogens (i.e. salmonella-shigella groups) could also be present. Esch coli is however widespread in nature and have their origin from faeces. Positive tubes at 37℃ only show the presence of Esch. coli that might have gone through mutation several times in the environment. Their presence however, particularly in small numbers, does not necessarily mean that the water contains faecal matter. This therefore suggests a low standard in the water treatment.
3. Results and Discussion
The results and interpretation of results are presented as follows:From Figure 1, the total depth gives 20.1m (60.33ft.) of resistivity of 34.1 Ώ-m and the 2 – D results showed the resistivities as 28.0 Ώ-m and 47.0 Ώ-m respectively(Figure 3 and Figure 4) showing that the study area has groundwater potential. Here the resistivity from the VES reading lies in between the resistivities gotten from the 2 – D results. From the study it can be seen that the depth to the aquifer is not too deep into the subsurface which may lead to easy and quick penetration of contaminants. The presence of coliform at count of 2.4×104and isolation of Escherichia Coli point to faecal contamination of sample and the presence of Staphylococcus aureus that are suspected from this study can percolate into the groundwater very quickly as a result of the direct contamination of storage tanks and pipes and also possibly from underground seepage having such depth.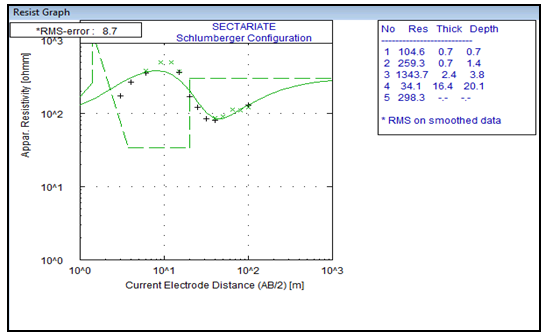 | Figure 1. Vertical Electrical Sounding (VES) Survey |
 | Figure 2. Study Area (around the building area) Map. Courtesy of Google satellite |
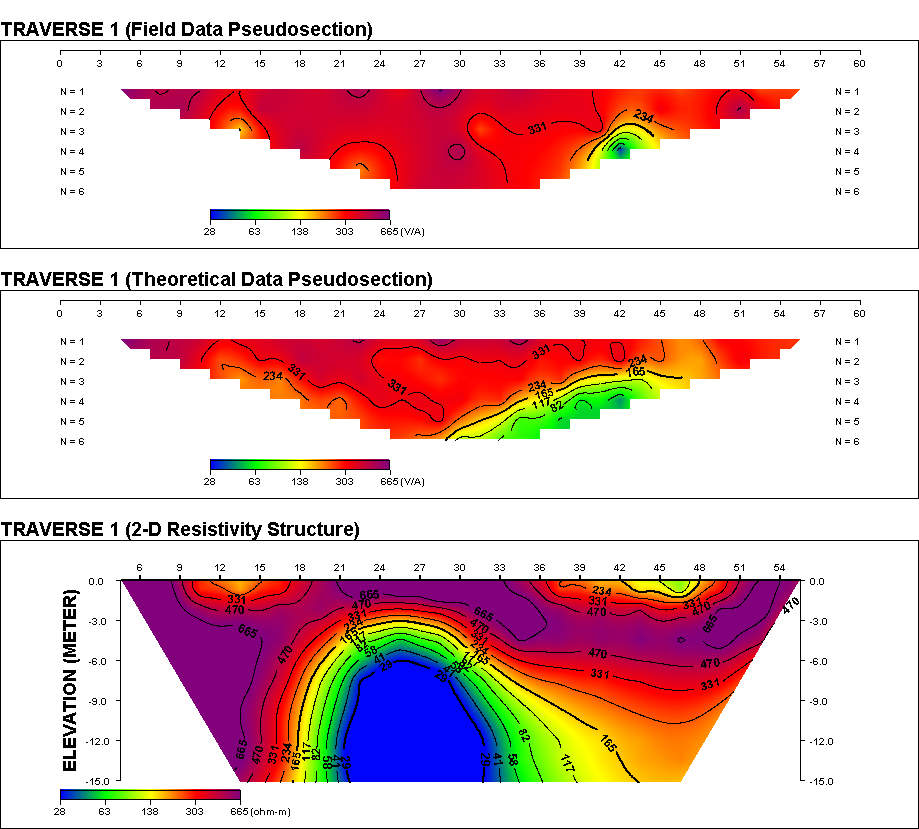 | Figure 3. 2 – D Resistivity Survey (Traverse 1) |
| | Sample Id | Rsd | Concentration | Corrected Concentration | Metals | | ADE | 10.3 | -0.2393C | -0.2393C | Lead | | ADE | >99 | -0.2331C | -0.2331C | Iron | | ADE | 13.1 | 0.0031 | 0.0031 | Nickel | | ADE | 37.8 | 0.0365 | 0.0365 | Copper |
|
|
3.1. Observation
It was observed that for all the water samples that was tested for the concentrations of heavy metals, all were within the WHO standard for drinking water. We therefore, advice that the authority in charge of water management in Ota, Nigeria to carry out Heavy metals assessment on groundwater to ensure that it is safe for domestic use.
3.2. Microbial Report
| Table 2. Morphological and Biochemical Characteristics of Bacterial Isolates |
| | Microbial Characteristics | Isolates | | 1 | 2 | | Colony MarginCell FormElevationTexturePigmentation | EntireCircularRaisedSmoothYellow | EntireCircularRaisedSmoothCream |
|
|
| Table 3. Cultural Characteristics |
| | Cultural Characteristics | Gram +ve | Gram -ve | | Cocci | Rods |
|
|
| Table 4. Biochemical Characteristics |
| | Biochemical Characteristics | Results | | Catalase | + | - | | Coagulase | + | - | | Coliform Test | - | A/G |
|
|
Table 5. Suspected Organism
 |
| |
|
3.3. Key
● +: Positive result● -: Negative result● A/G: Acid and Gas Production
3.4. Results
Total aerobic plate count: 2.3×106Coliform count: 2.4×104Organism Isolated: Escherichia ColiStaphylococcus aureus
3.5. Observations
● Total aerobic plate count of 2.3×106for drinking water is high and above standard specification● The presence of coliform at count of 2.4×104 and isolation of Escherichia Colipoint to faecal contamination of sample. This could be from underground seepage or direct contamination of storage tanks and pipes.● The presence of Staphylococcus aureuscould be from the environment of man● Finally, it will be advisable to treat the water to make it fit for human consumption.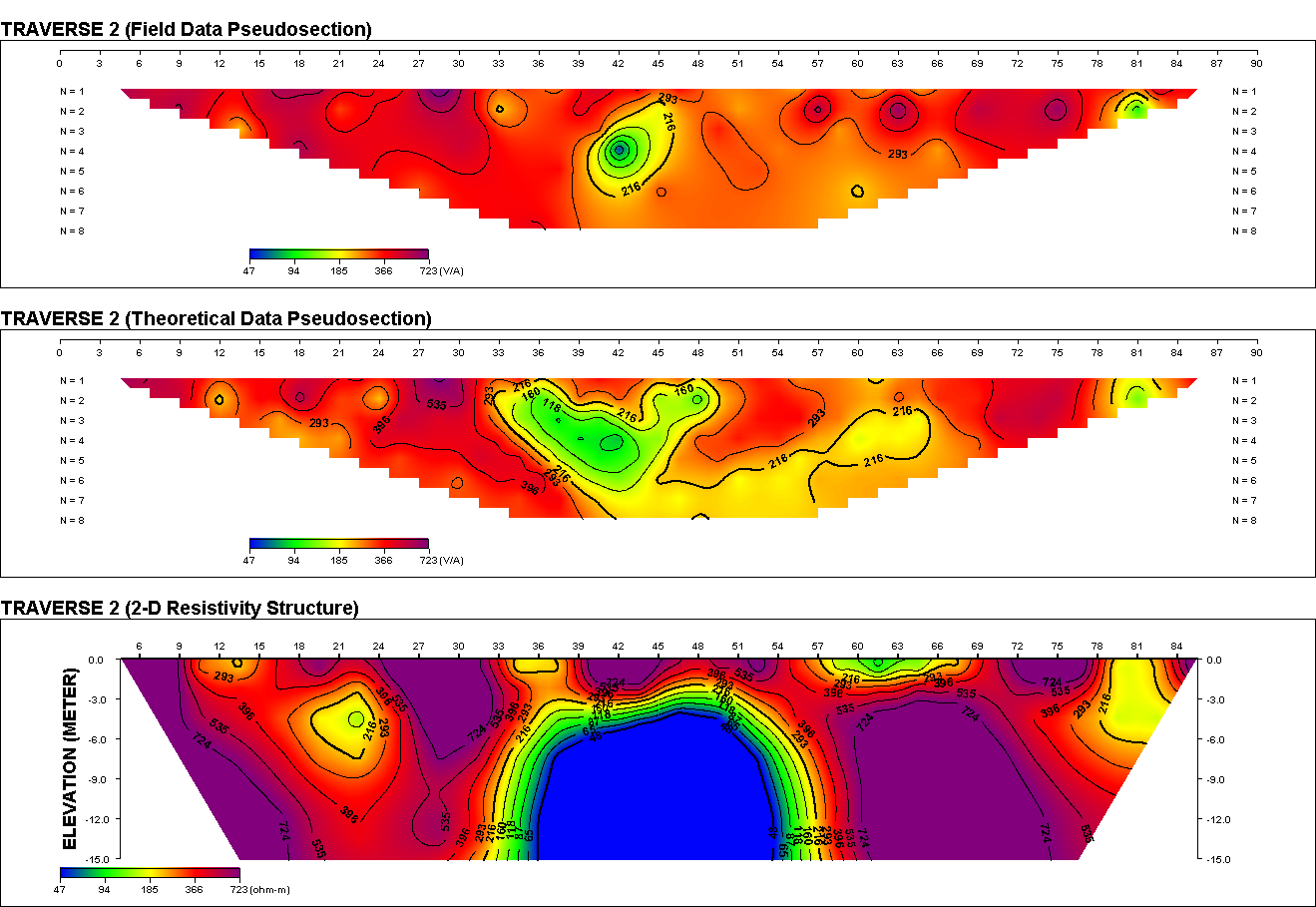 | Figure 4. 2 – D Resistivity Survey (Traverse 2) |
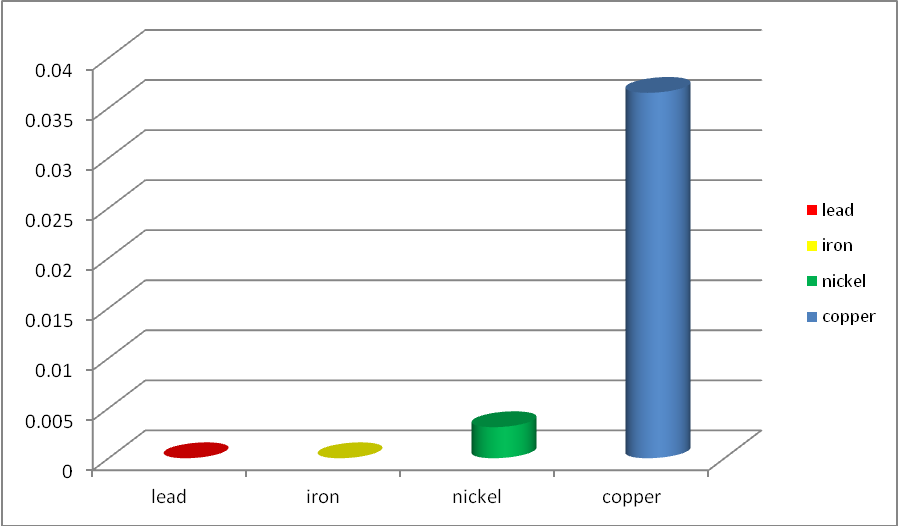 | Figure 5. Chart Showing Level of Heavy Metals in Samples |
4. Conclusions
From the VES results the depth to the aquifer lies around 20.1m (60.33ft.) of resistivity of 34.1 Ώ-m and the 2 – D results showed the resistivities as 28.0 Ώ-m and 47.0 Ώ-m respectively showing that the study area has groundwater potential. For the hydrochemical analysis on heavy metals, the result lies within World Health Organization (WHO) standard for drinking water. But for the microbial analysis, total aerobic plate count of 2.3×106 for drinking water is high and above WHO standard specification for drinking water. The presence of coliform at count of 2.4×104and isolation of Escherichia Colipoint to faecal contamination of sample is suspected. This could be from underground seepage or direct contamination of storage tanks and pipes. The presence of Staphylococcus aureus is also suspected.Geophysical investigation showed that the study area has groundwater potential and from the microbial analysis it is clear that the water need to be treated for domestic consumption. The borehole can also be relocated far away from the septic tank.
References
| [1] | Okosun, E.A. (1998): Review of the Early Tertiary Stratigraphy of Southwestern Nigeria. Journal of Mining and Geology.34, p. 27-35. |
| [2] | Herschy R.W. (1999): Hydrometry Principles and and Practices (2nd Edition) John Wiley & Sons, Chicchester. |
| [3] | Kaufmann, R. K. and Cleveland, C.J. (2008).Environmental Science, McGraw-Hill. p. 318-319. |
| [4] | World Health Organization. (2011). Guideline for drinking water quality – 4th Edition. ISBN 978 92 4 1548151. |
| [5] | World Health Organisation (1987). Guideline for Drinking Water, vol. 1. Recommendations. WHO, Geneva. |
| [6] | World Health Organization. (2004). Guidelines for Drinking water quality, Volume 1: 3rd edition, WHO Press, Switzerland. |
| [7] | Adepelumi, A. A., Ako, B. D. and Ajayi, T. R. (2001): Groundwater contamination in the basement-complex area of Ile-Ife, southwestern Nigeria: a case study using electrical resistivity method. Hydrogeology Journal, 9, p. 611-622. |
| [8] | Shalom N. C., Obinna C. N., Adetayo Y. O, Eze V. N. (2011). Assessment of water quality in Canaanland, Ota, Southwest Nigeria. Agriculture and biological journal of north america. (4): 577-583. |
| [9] | Rajini K., Roland P., John C., Vincent R. (2010). Microbiological and physiochemical analysis of drinking water in Georgetown, Guyana. Nature and Science, 8(8):261-265. |
| [10] | Osuinde.M.I. and Eneuzie, N.R. (1999). “Bacteriological analysis of ground water.”Nigeria Journal of Microbiology vol. 13:47-54 |
| [11] | Adekoya J.A, Williams A.B, Ayejuyo OO (2006). Distribution of heavy metals in sediments of Igbede, Ojo and Ojora rivers of Lagos, Nigeria. Environmentalist 26: 277-280. |
| [12] | Alile, O.M, Jegede, S.I. and Emekeme, R.E. (2009): Subsurface Probe and Hydrochemical Analysis for the Purpose of Sitting Waste Landfill. African Journal of Environmental Science and Technology Vol. 4(1), pp. 472-476. |






 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML