-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Geosciences
p-ISSN: 2163-1697 e-ISSN: 2163-1719
2012; 2(5): 125-132
doi: 10.5923/j.geo.20120205.04
Natural Radioactivity of Environmental Samples and their Impact on the Population at Assalamia-Alhomira Area in Yemen
Abd El-Hadi El-Kamel 1, Abdallah Ibrahim Abd El-mageed 1, Abd El-Bast Abbady 2, Shaban Harb 2, Imran Issa Saleh 3
1Department of Physics, Faculty of science, Assiut University, Egypt
2Department of Physics, Faculty of science, South Valley University, Egypt
3Department of Physics, Faculty of Education Toor El-Baha, Aden University, Yemen
Correspondence to: Abdallah Ibrahim Abd El-mageed , Department of Physics, Faculty of science, Assiut University, Egypt.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
Measurements of the levels of natural background level of the radioactivity from 226Ra and 232Th (and their decay progeny), as well as the primordial radionuclide 40K are the main objective of the current study. The present work investigated the radioactivity level of the rocks, soil and water samples at Assalamya-Alhomira area, Abyan district in Yemen. Thirty two rocks and soil samples and 3 groundwater samples from Assalamya-Alhomira area were analyzed by gamma-ray spectrometry using NaI(Tl) detector with specially designed shield. The concentration of three natural radionuclides namely 226Ra, 232Th, and 40K has been determined. The results showed that these radionuclides were present in concentrations of (48.68±6.7, 28.8±3.3 and 1618±53 Bq kg-1), (102.49±8, 149.8±8 and 2120±73 Bq kg-1) and (769.5±39, 4125±124 Bq kg-1 and 40K not detectable) for migmatitic biotite gneiss, psammitic and calc-silicate rocks, respectively. The highest activity concentration for 226Ra and 232Th is observed in calc-silicate rocks. From the microscopic study of the calc-silicate rocks we noted it consists of Calcite, Carbonate (Ca, Mg-CO3), diopside, magnetite and zircon minerals which accumulate in limited uranium and thorium. For clay and sandy soil the corresponding values were (41.46±5.6, 68.68±6 and 1224.7±31 Bq kg-1) and (80.77±4.5, 211.5±14 and 1004.8±40 Bq kg-1), respectively. Also radium equivalent activity, total dose rates and external hazard index of the (rocks/soil) samples in the area under consideration were calculated. The radioactivity concentration of 226Ra and 232Th for water samples ranged from 2.01±0.6 to 6.55±1.4 Bq l-1 and from 1.07±0.7 to 2.93±0.67 Bq l-1, respectively, while 40K activity was not detectable. The results showed that this area has a high background radiation due to the presence of Calc-silicate rocks which contain a high proportion of natural radioactive elements, and therefore represent a danger to the population lives in this area.
Keywords: Natural radioactivity, Rock, Soil, Calc-silicate rocks, Radionuclides
Article Outline
1. Introduction
- The radioactivity due to natural radionuclides in rocks, soil and water generate a significant component of the background radiation exposure to the population. The terrestrial component of the natural background is dependent on the compositions of the rocks, soil and water in which the natural radionuclides are contained[1]. Some areas are well known for their high background radiation like Rosetta in Egypt, the west cost of India and certain beaches in Brazil.Humans are exposed to natural terrestrial radiation that originates predominantly from upper 30 cm of the soil. Humans are also exposed by contamination of the food chain which occurs as a result of direct deposition of radionuclides on plant leaves, root uptake from contaminated soil or water and from direct ingestion of contaminated water. To assess these exposures radioactivity studies have been previously carried out in rock and soil samples in other part of the world, some similar to those by[2-12] and in water samples[13-20].The present work is the first one aims to suppose the radiological assessment program of Yemen, with the ultimate aim to establish a base line map of radioactivity background levels in Yemen environment. Assalamya-Alhomira area located some 300 km south east of Sana’a, the population of this area is about 553 people, and this area is known as high incidence of cancer cases among people. A total of 23 cases cancer diagnosed between 1990-2010, including (12 liver cancers, 5 stomach cancers, 2 brain cancers, 2 colon cancers, 1 bowel cancer and 1cervical cancer).This study deals with the natural radioactivity for rocks, soil and water in this area and assesses the radiological hazard resulting from them, using NaI(Tl) gamma-ray spectrometers. The absorbed dose rate, radium equivalent activities, external hazard index were evaluated and compared to the limits proposed by united nation scientific committee on the effect of atomic radiation[21].
2. Experimental
2.1. Geological Outline
- The study Assalamya-Alhomira area (~20 km2) is located some (300 km) southeast of Sana'a and located in Lat. 13º47'47''N and long 45º56'22''E. Assalamya-Alhomira area consist of Precambrian basement includes gneissic granite, pegmatite and biotitic gneiss[22]. Precambrian rocks are invaded by numerous calc-silicate bodies. Basement rocks are some of the oldest structures dating back to about 3 billion years. Magmata rocks, gneiss and schist rocks represent such structures. These structures appear as belts extending for tens of kilometers as ancient areas separating the small sheets which joined together and formed the Arab-African Shield[23].
2.2. Sampling and Sample Preparation
2.2.1. Rocks and Soil Samples
- A total of 23 rocks and 9 surface soil samples have been collected randomly from the studied area. Rock sample was crushed to small pieces and grinded to be powder. Soil samples were collected with the only constraint that no sampling site should be taken close to a field boundary, a road, a tree or other obstruction. Surface soils were then taken from different places randomly within cleared area from the ground surface up to 2 cm and mixed together thoroughly in order to obtain a representative sample of that area. Each sample (rock/soil) was dried in an oven at 105ºC and sieved through a 18 mesh which is the optimum size enriched in heavy mineral[24]. The samples were packed in plastic containers dimensions of 75 mm in diameter and 90 mm height. The samples were weighed and stored for a minimum period of one month to allow daughter products to come into radioactive equilibrium with their parents 226Ra and 232Th and then were counted for 12-24 hour depending on the concentration of the radionuclides.
2.2.2. Water Samples
- Because no running water in this area 3 groundwater samples were collected from the study area that used as drinking water. Measuring pH values as well as conductivity for water samples were measured in the laboratory. Standard polyethylene Marinelli beakers (1 liter) were used as a sampling and measuring container. Before use, the containers were washed with dilute hydrochloric acid and rinsed with distilled water. Each beaker was filled up to brim and a tight cap was pressed on so that the air was completely removed from it. The collected water samples were left for an overnight period in polyethylene containers to allow setting of any suspended solid materials and for each samples a clear supernatant was separated decantation. The clear solution was acidified by adding 0.5 ml of conc. HNO3 per liter, to prevent any loss of radium isotopes around the container walls, and to avoid growth of micro organisms[25]. The water samples were then homogenized well by shaking. The final acidity of water samples reaches pH-2. The samples were stored for over 30 days to reach secular equilibrium before radiometric analysis.
2.3. Experimental Setup
- Each sample was measured with a gamma-ray spectrometer consisting of a NaI(Tl) setup and multichannel analyzer 8192 channel, with the following specifications: resolution (FWHM) at 1.33 MeV 60Co is 60 keV – relative efficiency at 1.33 MeV 60Co is 7.5 %. The detector is shielded in a chamber of two layers starting with stainless steel (10 mm thick) and lead (30 mm thick). This shield serves to reduce different background radioactivity.To minimize the effect of the scattered radiation from the shield, the detector is located in the center of the chamber. Then the sample was placed over the detector for at least 10 h. The spectra were either evaluated with the computer software program Maestro (EG&G ORTEC), or manually with the use of a spread sheet (Microsoft Excel) to calculate the natural radioactivity. 226Ra activity of the samples was determined via its daughters (214Pb and 214Bi) through the intensity of the 295.22, 351.93 keV, for 214Pb Gamma-lines and 609.31, 1120, 1764.49 keV, for 214Bi Gamma-lines. 232Th activity of the sample was determined from the daughters (228Ac), (212Pb) and (208Ti) through the intensity of 209.25, 338.32, 911.2 keV Gamma-lines for (228Ac), (212Pb) emissions at 238.63 keV and (208Ti) emissions at 583.19, 2614 keV Gamma-lines. 40K activity determined from the 1460.7 keV emissions Gamma-lines.
2.4. Calculation of the Radiological Effects
- The most widely used radiation hazard index Raeq is called the radium equivalent activity. The radium equivalent activity is a weighted sum of activities of the 226Ra,232Th and 40K radionuclides based on the assumption that 370 Bq kg-1 of 226Ra, 259 Bq kg-1 of 232Th and 4810 Bq kg-1 of 40K produce the same gamma ray dose rate[26]. Radium equivalent activity can be calculated from the following relation suggested by Beretka and Mathew[27].
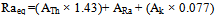 | (1) |
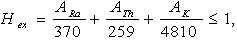 | (2) |
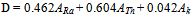 | (3) |
3. Results and Discussion
3.1. Natural Radioactivity in Rocks and Soil Samples
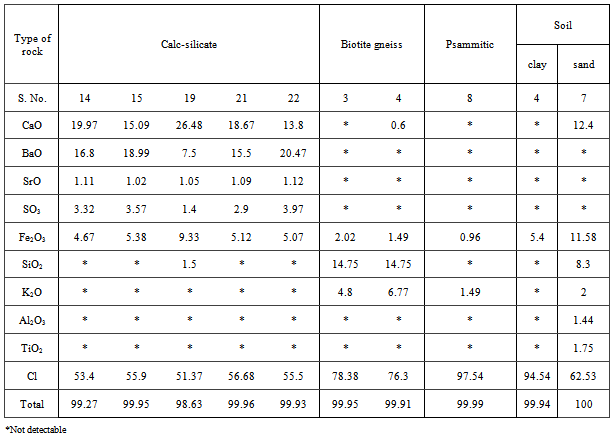
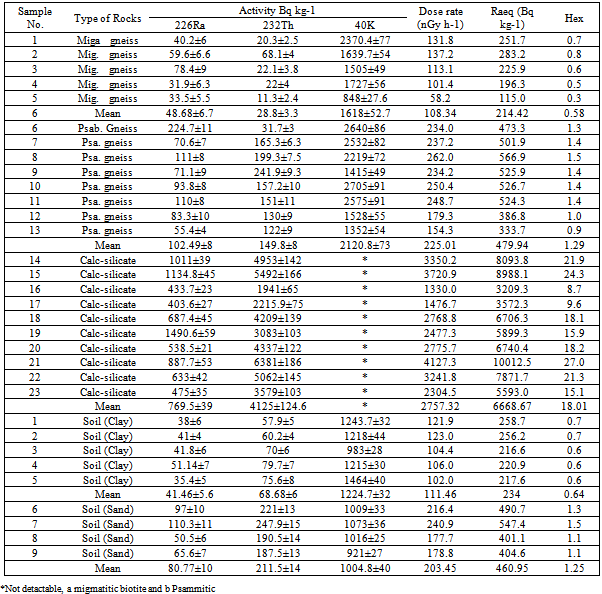
- Table 1 shows the chemical results of the rock analyses by XRF, The major oxides in rocks and soil samples are Cl, CaO, BaO, Fe2O3, SO3, SrO and K2O. The high values of U (226Ra) and 232Th measured in rocks can be attributed to the high positive correlation between these elements and CaO and Fe2O3, and negative correlation with Cl. Also the high values of 232Th measured in calc-silicate rocks can be attributed to the high positive correlation between thorium and BaO, SrO and SO3 which explains the higher values of thorium in calc-silicate rocks.Table 2 shows a summary of measurement for the activity concentration (Bq kg-1) of the natural radioactivity levels in rocks and soil due to 226Ra, 232Th and 40K. The table also lists the type of rock. The distribution of 226Ra, 232Th and 40K activity concentrations in all samples are given in Fig.2.
 | Figure 1. A map showing the study area |
|
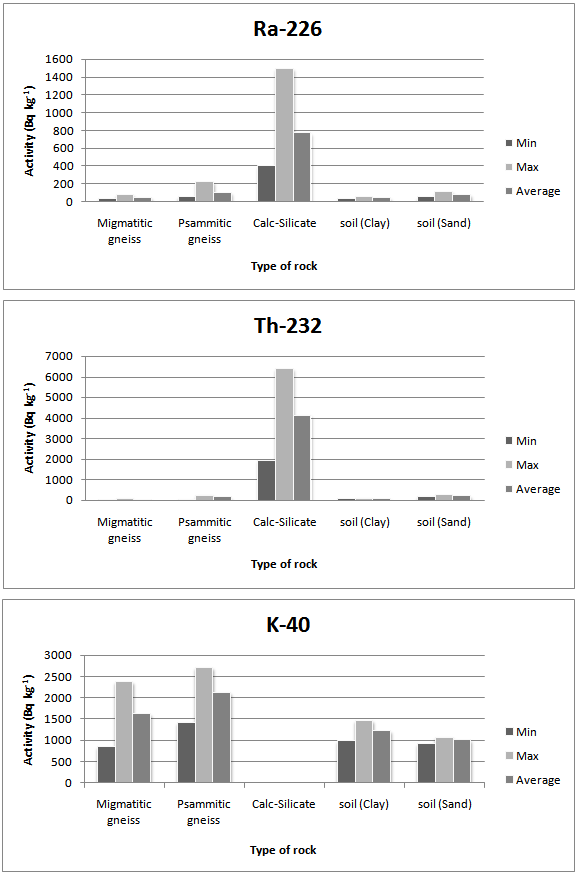 | Figure 2. Distribution of 226Ra, 232Th and 40K in Rocks and soil from Assalamya-Alhomira area in Yemen |
|
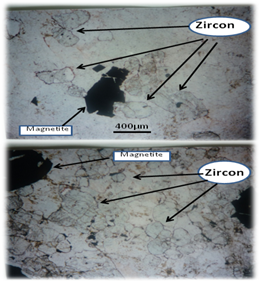 | Figure 3. Zircon and magnetite minerals encountered within calc-silicate rocks in Assalamya-Alhomira area |
3.1.1. Radiation Hazard Indices
- The absorbed dose rates in air 1.0 m above ground were calculated and given in table 1. The average absorbed dose rates for migmatitic biotite gneiss, psammitic and calc-silicate rocks are 108.34, 225.01 and 2757.32 nG h-1, respectively. According to the recent UNSCEAR reports the corresponding worldwide average values is 58 nG h-1. This reveals that the mean absorbed dose rates in air outdoors from these rocks are almost two, four and forty eight times higher than that of worldwide average value, respectively (Table 2).For soil the average absorbed dose rates in air are 111.46 and 203.45 nG h-1 for clay and sandy soil respectively. This means that the mean absorbed dose rates in air outdoors from soil approximately two and three past half times higher than that of worldwide average value.The calculated Ra-equivalent activities of the migmatitic biotite gneiss rocks are below the recommended value 370 Bq kg-1 as in[27] (Table 2), while the corresponding value for psammitic rocks is higher than recommended value (Table 2). For calc-silicate rocks the average Ra-equivalent activities is about eighteen higher than recommended value. For clay soil the corresponding value is below the recommended value but for sandy soil the Ra-equivalent value is higher than the recommended value (Table 2).The external hazard index (Hex) can be calculated from the equation 2 as in[27]. The results of Hex based on the criterion formula (Eq. (2)) are given in Table 2. The average values are 0.58, 1.29 and 18 for migmatitic biotite gneiss, psammitic and calc-silicate rocks, respectively. The recommended value is less than unity as in[21]. For soil the corresponding values of Hex are 0.64 and 1.25 for clay and sandy soil, respectively.When the results of this study for psammitic rocks are compared to the results of some of the previous studies ([3],[9],[10]) for granite rocks, it is clear that the results are less than those from Egypt, while similar to ([6],[11]) from Yemen and India. For calc-silicate rocks the results of this study is less than[2] from Brazil for the same type of rocks, while it is effectively superior to the values found in the literature for granite rocks. The values that were found for the sandy soil in the present study were superior to the global average as in[21], as well as to other studies ([6-8],[12]).
3.2. Natural Radioactivity in the Groundwater
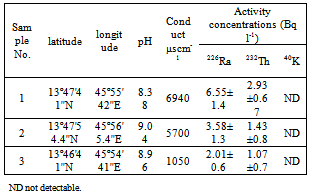
- The activity concentrations of 226Ra, 232Th and 40K in the groundwater that it used as drinking water in the study area were shown in table 3. The pH and conductivity can be also seen from table 3. As seen, all of the water samples have natural pH (8.38, 9.04 and 8.96) for sample 1, 2 and 3 respectively. The high alkalinity of water samples explain the high alkalinity of rocks in this area and reflect the high natural radioactivity in water samples.
|
4. Conclusions
- In the present study activity concentrations of 226Ra, 232Th and 40K for rocks, soil and water are evaluated. The average activity concentrations for migmatitic biotite gneiss and clay soil were inferior to the maximum recommended values (32, 45 and 412 Bq kg-1) as in[21]. On the other hand, the psammitic rocks presented 226Ra, 232Th and 40K average values are 102.49, 149.8 and 2120 Bq kg-1, which is about three times higher than the recommended limit. For calc-silicate rocks the corresponding values for 226Ra and 232Th are 769.5 and 4125 Bq kg-1, which are approximately 23 times higher the recommended values for 226Ra and approximately 85 times superior to the maximum recommended limit for 232Th. For sandy soil the activity concentration for 226Ra, 232Th and 40K are 80.77, 211.5 and 1004.8 Bq kg-1, which is about much higher than the recommended values. The results obtained for rocks and soil from this area are much higher than recommended limits and provide excessive exposure for inhabitants and cannot be used as construction materials with posing significant radiological threat to the population.Having those values to estimation the absorbed dose rate, radium equivalent and radiation hazard index, we can recommended in cases of psammitic, calc-silicate rocks and sandy soil should not be used as building materials, and considering the safety of the human population of studied area, it is suggested that the area should be avoided or seldom utilized.The groundwater data obtained in present study show the activity concentrations of 226Ra and 232Th ranged from 2.01 to 6.55 Bq l-1 and from 1.07 to 2.93 Bq l-1, respectively, While 40K was not detectable in these samples. The high activity concentrations for 226Ra and 232Th measured in water samples explain the relationship between the groundwater and calc-silicate rocks in the region, for this reason we suggests the investigated groundwater are not acceptable as drinking water.Therefore, the present study has pointed out the area under study need further studies in order to better understand the origin and distribution of naturally occurring radionuclides.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML