-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Frontiers in Science
p-ISSN: 2166-6083 e-ISSN: 2166-6113
2021; 11(1): 18-23
doi:10.5923/j.fs.20211101.02
Received: Oct. 14, 2021; Accepted: Nov. 14, 2021; Published: Nov. 26, 2021

Effect of Absorption Selenium (Se) on Mycelium Growth of Pleurotus ostreatus Mushrooms
Tarek Fekry1, Amal A. AbdElaziz1, Shaden Muawia1, Yahya M. Naguib2, Hany Khalil1, Mohamed F. Salem3
1Department of Molecular Biology, Genetic Engineering and Biotechnology Research Institute, University of Sadat City, Sadat City, Egypt
2Medical Physiology Departments, Faculty of Medicine, Menofyia University, Egypt
3Department of Environmental Biotechnology, Genetic Engineering and Biotechnology Research Institute, University of Sadat City, Sadat City, Egypt
Correspondence to: Hany Khalil, Department of Molecular Biology, Genetic Engineering and Biotechnology Research Institute, University of Sadat City, Sadat City, Egypt.
| Email: |  |
Copyright © 2021 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Selenium (Se) is a critical micronutrient for a variety of human health issues, included cardiovascular health, neurodegeneration and cancer prevention, and appropriate immunological responses. Selenium in the form of sodium selenite (Na2SeO3) was measured in the culture medium of Pleurotus ostreatus at concentrations of 2.0 mg/L, 5.0 mg/L, 10.0 mg/L, 20.0 mg/L, 40.0 mg/L, and 60.0 mg/L. It is great worthy to mention that as the concentration of Se increased, the amount of biomass produced. At a Se concentration of 20.0 mg/L, biomass reduced from 5.56 g/L in the control to 3.20 g/L, whereas production was entirely suppressed at concentrations of (40.0 and 60.0) mg/L. Even though colony diameters were nearly comparable in the control and media enriched with the lowest three Se concentrations, biomass output was lower at all three Se concentrations compared to the control.
Keywords: Selenium (Se), Pleurotus ostreatus, Mushrooms
Cite this paper: Tarek Fekry, Amal A. AbdElaziz, Shaden Muawia, Yahya M. Naguib, Hany Khalil, Mohamed F. Salem, Effect of Absorption Selenium (Se) on Mycelium Growth of Pleurotus ostreatus Mushrooms, Frontiers in Science, Vol. 11 No. 1, 2021, pp. 18-23. doi: 10.5923/j.fs.20211101.02.
1. Introduction
- Mushrooms are fungi, which means they lack chlorophyll. They are typically saprophytic in nature. That is, they obtain sustenance through decomposing non-living organic material [1]. Mushrooms are becoming increasingly popular as a source of nutraceuticals, antioxidants, prebiotics, immunological regulating, anti-inflammatory, cardiovascular, anti-microbial, and anti-diabetic characteristics. [2,3]. Pleurotus ostreatus is the binomial name. Pleurotus is a genus of roughly 40 species of mushrooms that are popularly referred to as "oyster mushrooms".Several species of Pleurotus have a considerable commercial value in the world market for edible farmed mushrooms at the moment [4]. Pleurotus species have important amino acids including arginine, glutamine, and glutamic acid, as well as vitamins and minerals. [5]. Due to its involvement in a variety of key enzymes and proteins, selenium is a necessary microelement for proper human and animal growth and development in minimal amounts. Oyster mushroom mycelium has bioremediation capabilities, meaning it can treat soil polluted with oil derivatives, polycyclic aromatic hydrocarbons, or heavy metals. Pleurotus cultivation can therefore tackle one of the most pressing issues in soil waste management, generate economic benefits, and safeguard the environment. [6].High Se concentrations can prevent Pleurotus ostreatus mushrooms from growing and producing mycelium. [5,7]. As a result, it would be worthwhile to investigate the effects of various Se chemical forms on the development and morphology of P. ostreatus mycelium to determine the best concentration to use for enrichment. P. ostreatus is also one of the world's most frequently cultivated and consumed mushrooms. [8], demonstrating its economic and nutritional significance.Humans are beginning to recognize selenium as a necessary nutrient. Selenium and its compounds are found in foods in the form of selenoamino acids, selenoproteins, selenide, and selenite, and have been shown to have biological effects through iodothyronine deiodinase, glutathione peroxidases, phospholipid hydroperoxide, sperm capsule, selenoprotein, and thioredoxin. Selenium compounds inhibit tumor-promoting signaling enzymes including protein kinase C (PKC) and act as antioxidants via selenoproteins and thioredoxin reductases. [9]. The International Food and Nutrition Board recommends a daily dose of 40–70 g selenium for males and 45–55 g selenium for women, and 25 g selenium for children [10]. Se can protect against chronic illnesses such as cancer, cardiovascular disease, oxidative stress, and inflammatory disorders. Food, on the other hand, has a low Se content. [11].
2. Materials and Methods
- Organism and Cultivation ConditionsThe culture of P. ostreatus originated and was identified by Dr. Mohamed Fathy Salem, GEBRI, Sadat City, Egypt. P. ostreatus maintained in Petri dishes containing Potato-Dextrose Agar (PDA) culture medium, pH 5.8. Petri dishes were containing sterilized medium were inoculated with a one-disc of mycelia (about 1 cm2 each) and incubated at 25±2°C for 7 days, then stored in a refrigerator at 4°C.Submerged culture medium for mycelium The following was the culture media (g/L distilled water): (glucose, 10 g/L; NH4NO3, 2 g/L; K2HPO4, 1 g/L; NaH2PO4, 0.4 g/L; MgSO4, 0.5 g/L; yeast extract, 2 g/ L; pH 6.5). 1) Selenium was tested in the form of sodium selenite (Na2SeO3) at concentrations of 2.0 mg/L, 5.0 mg/L, 10.0 mg/ L, 20.0 mg/L, 40.0 mg/L and 60.0 mg/ L. In addition, added to a sterilized modified synthetic medium that was optimum for biomass production in 400-mL Erlenmeyer flasks containing 100.0 mL modified synthetic medium; as a control, medium without Se was utilized. For each Se concentration, three replicas were created. 2) Incubation at room temperature (25°C) for 7 days on a rotary shaker (100 rpm). 3) Sterile distilled water washing of acquired biomass (3times). 4) Biomass homogenization in a laboratory blender with 100 mL of sterile dH2O. Selenium (Se) determinedThe concentration of absorbed Se was measured using an HG-AAS Model SP190 hydride generation atomic absorption spectrophotometer (Pye Unicam, England). 0.1 g of dried biomass was dissolved in 10.0 mL concentrated HNO3 and 3.0 mL concentrated HCl, then diluted with Milli-Q water to a final volume of 20.0 mL. A standard curve was created using solutions containing Se at concentrations of 0.0 g/L, 10.0 g/L, 25.0 g/L, and 50.0 g/L. Data on absorbed Se from the initial incubation medium is reported as a percentage of total absorbed Se and as g of absorbed Se per g of dried biomass. Statistical AnalysisThe mean standard error of measurements from triplicate experiments is used to express the data. Using STATISTICA software, version 5.0, a one-way analysis of variance (ANOVA) was conducted to evaluate the significance of differences among the absorbed Se concentrations (StatSoft, Inc.). Significant values were defined as those smaller than 0.01.
3. Results and Discussion
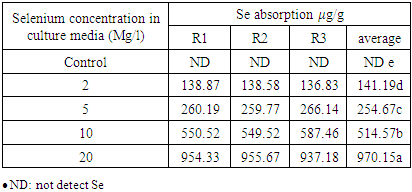
- Mycelium growth and absorption capability are determined as a function of selenium concentrations. Mycelium growth was good and growth intensity was comparable to that of the control medium in media enriched with Se at concentrations of 2.0 mg/L, 5.0 mg/L, and 10.0 mg/L. Se concentrations of 20.0 mg/L were less favorable for development, resulting in lower mycelium density; Se concentrations of 40.0 mg/L and 60.0 mg/L significantly hampered growth, whereas higher Se concentrations completely stopped it. As the concentration of Se increased, the amount of biomass produced decreased. At a Se concentration of 20.0 mg/L, biomass reduced from 5.56 g/L in the control to 3.20 g/L, whereas production was entirely suppressed at concentrations of (40.0 and 60.0) mg/L (Figure 1).
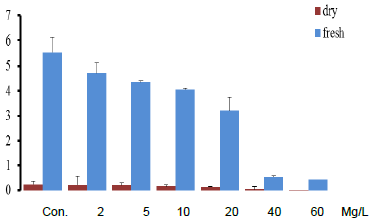 | Figure 1. Mycelia growth rate (gram) for Selenium concentration in culture media (mg/L) |
|
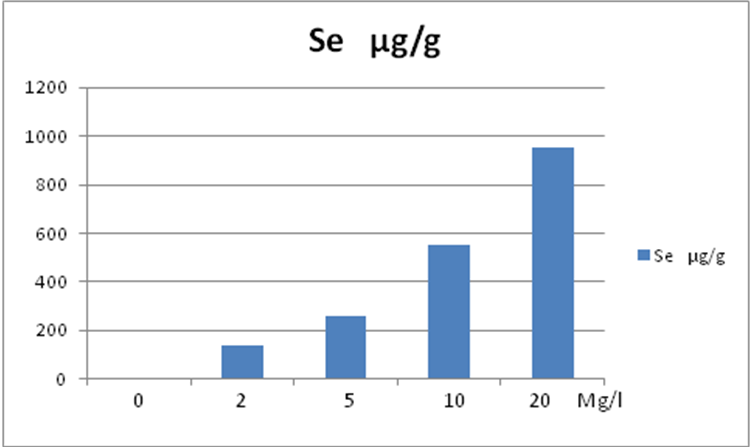 | Figure 2. Se absorption µg/g for Selenium concentration in culture media (mg/L) |
|
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML