-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Frontiers in Science
p-ISSN: 2166-6083 e-ISSN: 2166-6113
2020; 10(1): 1-6
doi:10.5923/j.fs.20201001.01

Evaluation of Comparative Phenolic Contents and Antioxidant Activity of Mikania Species Available in Bangladesh
Rafeza Khatun1, Mamunur Rashid2, AHM Khurshid Alam2, Young-Ik Lee3, Md Aziz Abdur Rahman2
1Department of Pharmacy, Comilla University, Comilla, Bangladesh
2Department of Pharmacy, University of Rajshahi, Rajshahi, Bangladesh
3Lee’s Biotech Co., Korea Research Institute of Bioscience and Biotechnology, Daejeon, Korea
Correspondence to: Md Aziz Abdur Rahman, Department of Pharmacy, University of Rajshahi, Rajshahi, Bangladesh.
| Email: |  |
Copyright © 2020 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This paper represents comparative phenolic contents and antioxidant activity of available Mikania species (family-Asteraceae) in Bangladesh with folkloric reputation. These species are Mikania cordata (MC), Mikania micrantha (MM) and Mikania scandens (MS). The ethanolic extract (70%) of the selected plants were tested for total phenolics (TPC), total flavonoids (TFC), total antioxidant capacity (TAC), ferrous reducing capacity (FRC), DPPH free radical scavenging assay (DRSA), hydroxyl radical scavenging activity (HRSA) and hydrogen peroxide Assay (HPSA). The TPC of MC, MM and MS were 456.3±0.08, 271.2±0.80 and 168.1±0.83 mg of gallic acid equivalent/g of dry extract and TFC were 672.8±0.76, 349.5±0.97 and 152.8±0.76 mg of catechin equivalent/g of dry extract, respectively. The data indicated that MC contained higher amount of TPC and TFC followed by MM and MS. The TAC and FRC were in the following order: MC> MM>MS. In DRSA, the IC50 of MC was 11.50 μg/mL which was comparable to standard ascorbic acid (10.01 μg/mL) followed by MM (19.76 μg/mL) and MS (36.39 µg/mL). Same results were observed for HRSA and HPA where MC showed the most potent inhibitory activity. The TPC of MC was also positively correlated (p< 0.001) with DFSA, HRSA and HPSA. The findings conclude that Mikania cordata possesses highest antioxidative phytochemicals compare to others available mikania species, justifying the frequency of use in traditional folk medicine in Bangladesh compared to other species.
Keywords: Mikania cordata, M. micrantha, M. scandens, Asteraceae, Antioxidant, Polyphenols
Cite this paper: Rafeza Khatun, Mamunur Rashid, AHM Khurshid Alam, Young-Ik Lee, Md Aziz Abdur Rahman, Evaluation of Comparative Phenolic Contents and Antioxidant Activity of Mikania Species Available in Bangladesh, Frontiers in Science, Vol. 10 No. 1, 2020, pp. 1-6. doi: 10.5923/j.fs.20201001.01.
Article Outline
1. Introduction
- Antioxidants play an important role in delaying or preventing degenerative diseases caused by oxidative damage of living cell components by stabilizing or deactivating free radicals [1-2]. Primary antioxidants are mainly chain breakers, able to scavenge radical species by hydrogen donation. Phenolic compounds from medicinal herbs and dietary plants include flavonoids, tannins, coumarins, xanthenes, etc. have been shown to scavenge radicals and therefore are viewed as promising therapeutic drugs for free radical pathologies [3]. Medicinal plants have been used for thousands of years in folk medicines in Asian and African populations and many plants are consumed for their health benefits in developed nations. Moreover, there is a less preference for antioxidants from synthetic source [4] due to the possibility of adverse effects in humans, without additional nutritional benefits. Keeping this in view, Mikania species that are well-known folkloric reputed tropical medicinal plants in the Asteraceae family and available in Bangladesh were selected for investigation. There are approximately 430 species of this genus and only 12% have been studied [5]. Commonly available Mikania species in Bangladesh are Mikania cordata, Mikania micrantha, and Mikania scandens (family: Asteraceae). The species are used as ethnomedicine in treating wounds, indigestion, dysentery, gastrointestinal sore, coughs, eye sores, etc [6] and a wide variety of biological activities such as antimicrobial, anti-inflammatory, antispasmodic, antitumoral, anticoagulant, bronchodilator and antioxidant activity are reported from Mikania cordata and Mikania micrantha [5,7-9]. Also, the plant Mikania cordata has been used in traditional medicine of Bangladesh to treat inflammations, tumor and infectious diseases by folklore people [6,9-10]. Phytochemical studies have reported diverse chemicals from the species. The main chemical groups are coumarins and derivatives, sesquiterpenes, sesquiterpenes, lactones, diterpenes, phytosterols, terpenoids and flavonoids [6].Though a lot of research have been done on Mikania species but most of them are limited to Mikania micrantha and Mikania cordata, and comparative evaluation of antioxidant activity that might be interesting among biological scientists was not reported elsewhere. In our previous study we reported comparative antimicrobial evaluation of three available Mikania species in Bangladesh [7]. In this paper we report comparative phenolic contents and antioxidant activity of M. cordata, M. micrantha, and M. scandens.
2. Materials and Methods
2.1. Collection, Identification and Extraction
- The whole part of M. cordata (MC), M. micrantha (MM) and M. scandens (MS) were collected from Rajshahi (northern part of Bangladesh), Barisal (Southern part) and Kushtia (western part), respectively, during the month of August 2017 and were identified by Dr. AHM Mahbubur Rahman, associate professor and taxonomist, Department of Botany, University of Rajshahi, Bangladesh. The plants were labelled, air dried for several days and then oven dried at 45°C for 24 hours. The dried plants were crushed separately into course powder. About 170 gm powdered plant materials were taken separately in an amber coloured extraction bottle and were soaked with 70% ethanol (90 mL × 3 times) for 7 days with occasional shaking. The extracts were filtered through cotton and Whatman No. 1 filter, concentrated with a rotary evaporator under reduced pressure at 45°C and preserved at 4°C. The percentage of MC, MM and MS extract (w/w) were11.00%, 10.5% and 9.3%, respectively.
2.2. Chemicals
- DPPH, KOH, H2O2, K3[Fe(CN)6], CH3COOK, phosphate buffer, catechin (CA), (NH4)2Fe(SO4)2·6H2O, butylated hydroxytoluene (BHT), gallic acid (GA), ascorbic acid (AA), AlCl3, Trichloro acetic acid (TCA), Na3(PO4), ammonium molybdate, DMSO, EDTA, thiobarbituric acid (TBA), acetyl acetone and FeCl3 were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Folin–Ciocalteu reagent and Na2CO3 were obtained from Merck (Damstadt, Germany).
2.3. Estimation of Total Phenolics
- Total phenolic contents (TPC) of the extracts were determined by the modified Folin-Ciocalteu method [11]. An aliquot of extract was mixed with diluted 2 mL Folin-Ciocalteu reagent and 2 ml (75 g/L) of Na2CO3. The tube was vortexed for 15 seconds and allowed to stand for 20 minutes at 25°C for color development. Absorbance was measured at 760 nm using UV-spectrophotometer (Shimadzu, USA). TPC was expressed in terms of gallic acid equivalent, GAE (mg of GA/g of dry extract).
2.4. Determination of Total Flavonoids
- Total flavonoids (TFC) were estimated using the method described by Ordonez et al. [12]. To 0.5 mL of sample, 1.5 ml of MeOH, 100 μl of 10% AlCl3, 100 μl of 1M potassium acetate solution and 2.8 ml of distilled water were added. After 90 minutes of incubation at RT, the absorbance was measured at 420 nm. TFC was expressed in terms of catechin equivalent, CAE (mg of CA/g of dry extract).
2.5. Determination of Total Antioxidant Capacity
- Total antioxidant capacity (TAC) was determined by the method reported by Prieto et al. [13] with some modifications. 0.5 mL of samples at different concentrations was mixed with 3 mL of mixture containing 0.6 M H2SO4, 28 mM Na3PO4 and 1% ammonium molybdate. The mixtures were incubated at 95°C for 10 minutes and the absorbance were measured at 695 nm Increased absorbance of the mixture indicated increase total antioxidant capacity.
2.6. Determination of Ferrous Reducing Capacity
- The ferrous reducing capacity (FRC) was evaluated by the method of Oyaizu [14]. In 0.25 mL samples of different concentrations, 0.625 mL of potassium buffer (0.2 M) and 0.625 mL of 1% [K3Fe (CN)6] solution was added and the mixture was incubated for 20 minutes at 50°C. Then 0.625 mL of 10% TCA solution was added and the mixture was centrifuged at 3000 rpm for 10 minutes. After which, 1.8 mL supernatant was withdrawn and mixed with 1.8 mL of distilled water and 0.36 mL of 0.1% FeCl3 solution. The absorbance was measured at 700 nm. Increased absorbance of the mixture indicated increase reducing capacity.
2.7. DPPH Free Radical Scavenging Assay
- Free radical scavenging activity (DRSA) was determined by DPPH free radical scavenging assay method [15]. A solution of 0.1 mM DPPH in MeOH was prepared and 2.4 mL of this solution was mixed with 1.6 mL of extractives in MeOH at different concentrations. The mixture was vortexes thoroughly and left in the dark for 30 minutes. The absorbance was measured at 517 nm. Percentage of DRSA was calculated by: % DRSA = [ (A0-A1) ̸ A0]×100, where A0 and A1 are absorbance of control and extractives, respectively. IC50 was calculated from Percentage of inhibition vs concentration curve.
2.8. Hydroxyl Radical Scavenging Activity
- Hydroxyl radical scavenging activity (HRSA) was determined by the method of Klein et al. [16] with a slight modification. 1 mg of extractive (in 1 mL of 70% MeOH) was taken in test tube. 100 µL of 28 mM 2-deoxy D-ribose, 500 µL of extract, 100µL of 1.04 mM EDTA, 100 µL of 1.0 mM H2O2, 100 µL of 1mM ascorbic acid and 100 µL of 200 µm FeCl3 were mixed together and incubated at 37°C for 1hr. 1% TBA and 10% TCA, 1 mL each were added in each test tube and incubated the mixture at 100°C for 20 minutes. Absorbance was taken at 532nm. % HRSA = [(A0-A1)/A0] ×100, where A0 and A1 are the absorbance of control and extractive, respectively. IC50 was calculated from Percentage of inhibition vs concentration curve.
2.9. Hydrogen Peroxide Assay
- The ability of crude extracts to scavenge H2O2 (HPSA) was assessed by the method described by Zhang [17]. Two different concentrations 250 µg/mL and 125µg/mL of extract were prepared in MeOH. Aliquot of 1.0 mL H2O2 (0.1 mM) and 1.0 mL of extract were mixed followed by adding 2 drops of 3% ammonium molybdate. 10 mL of 2M H2SO4 and 7.0 mL of 1.8 M KI were added and the mixture was titrated with 5.09 mM Na2S2O3 until disappearance of yellow color. Calculated % HPSA = [(V0-V1)/ V0] × 100, where, V0 and V1 are volume of Na2S2O3 for blank and for extract, respectively.
2.10. Statistical Analysis
- All values were expressed as mean ± Standard Deviation (SD). Statistical comparison was performed by One-way analysis of variance (ANOVA), followed by using Dunnet test. Results were considered as significant when p values less than 0.05 (p<0.05).
3. Results and Discussion
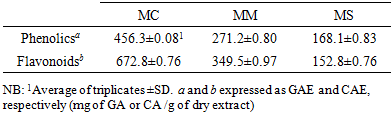
3.1. Total Antioxidant Capacity (TAC) and Ferrous Reducing Capacity (FRC)
- The TAC of MC, MM and MS are shown in Figure 1A. Among these extractives, MC showed significant activity compared to standard CA. The activity of MC is significantly higher (p<0.01) than that of MM and MS. The extractives were found to increase the total antioxidant activity with the increasing concentration of the sample. At 100µg/mL, the absorbance of MC, MM, MS and standard CA were 0.5130, 0.456, 0.411 and 0.945 respectively.The FRC of MC, MM and MS are shown in Figure 1B. At 100 µg/mL, the absorbance was 1.434, 0.988, 0.826 and 3.432 for MC, MM, MS and CA, respectively. The reducing activity increased with the increasing concentration of the extracts and a higher absorbance indicates a higher reducing power. Among the extractives, MC showed highest reducing activity whereas MS showed lowest activity compare to standard ascorbic acid. The total antioxidant potential and ferrous reducing capacity indicate the ability to reduce Mo (VI) to Mo (V) and to reduce Fe3+ to Fe2+, respectively. Both of the tests indicate the presence of phenolic compounds which might act as electron donors. Strong activity of MC is due to presence of highest concentration of polyphenols [18].
 | Figure 1. Determination of A) TAC and B) FRC of MC, MM and MS |
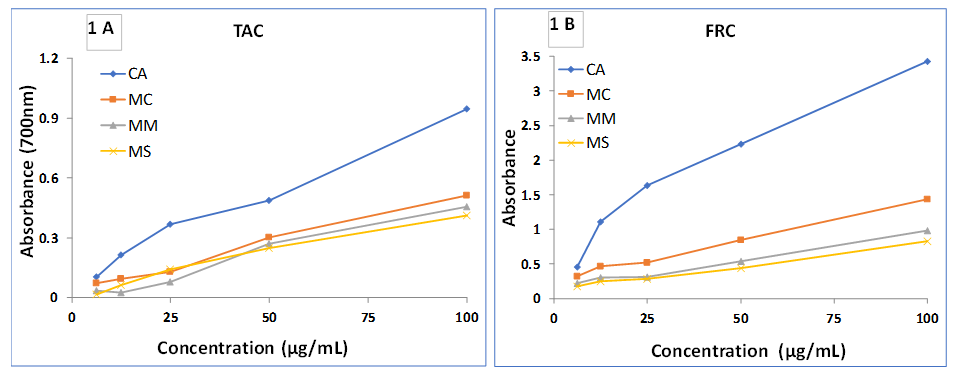
3.2. DPPH Free Radical Scavenging Assay (DRSA)
- Figure 2A shows DRSA of MC, MM and MS. Among the three species, the highest scavenging activities was found in MC followed by MM and MS. The IC50 of MC, MM, MS and standard AA were found to be 11.50, 19.76, 36.39 and 10.01 µg/mL, respectively. DPPH assay is based on the ability of 1, 1 diphenyl-2-picryl-hydrazyl (DPPH) to accept electron in the presence of antioxidants [19-20]. The results obtained in this investigation reveal that all extractives are free radical scavengers, which might be attributed to their electron donating ability.
3.3. Hydroxyl Radical Scavenging Activity (HRSA)
- Figure 2B shows HRSA of the extracts. At 100 µg/mL, the percentage of scavenging activity of MC, MM, MS and standard CA were 87.55, 78.22, 66.23 and 96.64%, respectively. The results indicate significant scavenging activity of MC compare to standard CA.Hydroxyl radicals are the major reactive oxygen species (ROS) causing lipid oxidation and enormous biological damage [21]. The process of lipid peroxidation is mediated by the interaction of hydroxyl radicals with the cell membrane; subsequently producing lipid-derived free radicals [22]. The results obtained in this study revealed that MC showed significant ability to quench hydroxyl radicals, hence a better source to prevent lipid peroxidation compare to MM and MS.
 | Figure 2. Determination of A) DRSA and B) HRSA of MC, MM and MS |
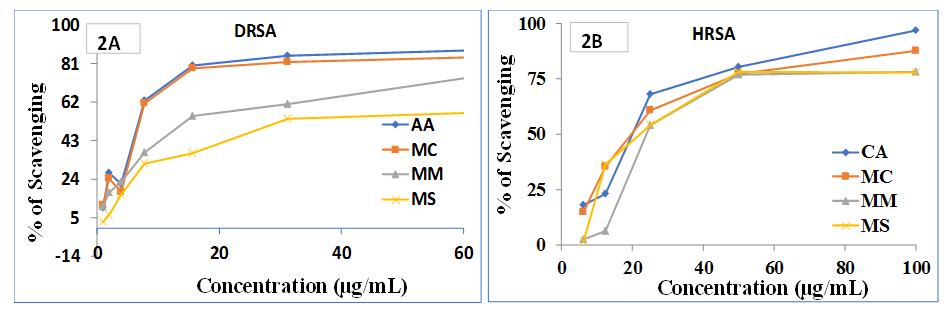
3.4. Hydrogen Peroxide Assay (HPSA)
- The result is shown in Figure 3. Among the extractives, activity of MC was closely resembled to that of standard. Other extracts showed moderate H2O2 scavenging activity compare to the standard BHT. At 125 µg/mL, percentage of scavenging of MC, MM, MS and BHT was found to be 44.28, 26.71, 12.85 and 50.48% while at 250 µg/mL the activity was 71.42, 61.42, 32.42 and 80.57%, respectively.Oxidative stresses play harmful physiological responses which may lead to develop cell damages and various diseases such as diabetes, atherosclerosis, ischemic injury, inflammation and carcinogenesis [23]. Hydrogen peroxide is one of these which may produce oxidative stress and finally free radical. Our study demonstrates that among the extractives MC is able to scavenge hydrogen peroxide significantly compare to MM and MS.
 | Figure 3. Percentage of scavenging activity of MC, MM and MS in HPSA |
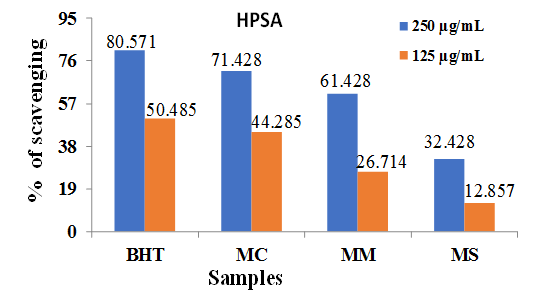
3.5. Total Phenolic (TPC) and Flavonoid (TFC) Content
- Table 1 shows the total phenolic and flavonoid contents of MC, MM and MS. The results showed that MC contains highest conc. of polyphenols followed by MM and MS.
|
3.6. Correlation of Total Phenolics with Antioxidant Activity
- Figure 4 represents the correlation and regression (p-value < 0.001) of TPC with % DRSA (Figure 4A), % HRSA (Figure 4B) and % HPSA (Figure 4C). Significant correlations (p-value < 0.001) were observed for all the extractives.
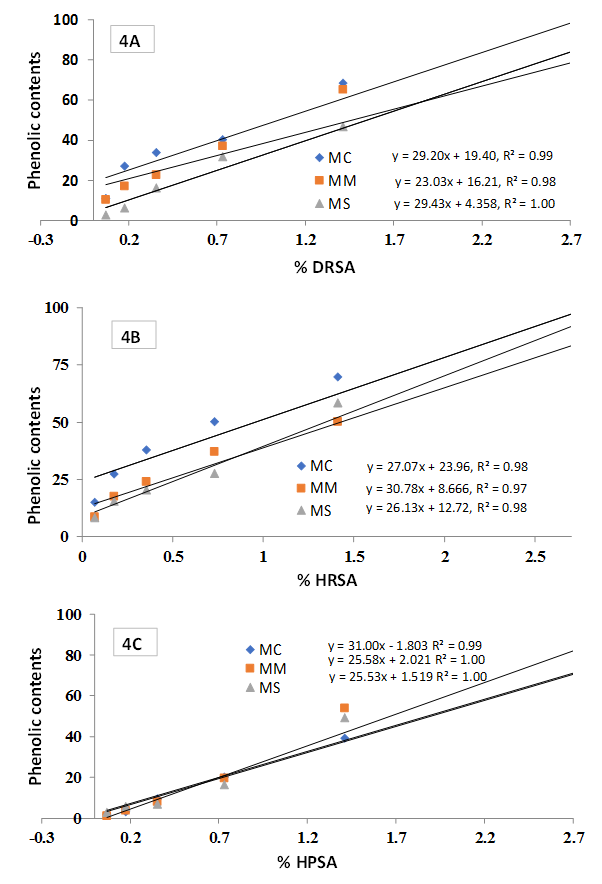 | Figure 4. Relationship of Phenolic content with A) % DRSA, B) % HRSA and C) % HPSA. Date expressed as mean ± SD (n=3, p< .001) |
4. Conclusions
- The plant M. cordata, M. micrantha and M. scandens have been used to treat a variety of diseases in Bangladesh as traditional medicine. Compared to the effects on different diseases, little is known about the comparative antioxidant activities of these species. The present study revealed the usefulness of M. cordata, M. micrantha and M. scandens against oxidative stress. Among these, M. cordata showed noticeable antioxidant activity against DPPH, hydroxyl radical assay, hydrogen peroxide assay, total antioxidant capacity and ferrous reducing capacity assay. The significant antioxidant activity of M. cordata was due to presence of highest phenolic and flavonoids contents. A direct correlation between antioxidant capacity and phenolic contents of some plant extracts has been previously reported [26]. Our designed study also revealed a direct correlation of polyphenolics with antioxidant activity. The overall results justify the using of M. cordata in traditional folk medicine in Bangladesh compared to other species. The high variability in antioxidant activity among different species may contribute equally to variability of Mikania composition, hence, interdisciplinary research and the development of modern combinatorial techniques make possible the discovery of novel agents from these species. Further studies on the effective antioxidants contained species and the mechanisms by which they protect against disease development are highly recommended.
ACKNOWLEDGEMENTS
- The authors acknowledge the Department of Pharmacy, University of Rajshahi, Bangladesh for financial support. We also thank to Dr. AHM Mahabubur Rahman, Assistant Professor, Department of Botany, University of Rajshahi, Bangladesh for identifying the plant.
List of Abbreviations
- AA: Ascorbic acid; CAE: Catechin equivalent; DPPH: 1, 1- diphenyl-2-picrylhydrazine; GA: Gallic acid; GAE: Gallic acid equivalent; OS: Oxidative stress; ROS: Reactive oxygen species; MC: Mikania cordata; MM: Mikania micrantha, MS: Mikania scandens; TAC: Total antioxidant capacity; TCA: Trichloro acetic acid; TPC: Total phenolics; TFC: Total flavonoids; TAC: Total antioxidant capacity; FRC: Ferrous reducing capacity; DRSA: DPPH free radical scavenging assay; HRSA: Hydroxyl radical scavenging activity; HPSA: Hydrogen peroxide Assay.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML