-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Frontiers in Science
p-ISSN: 2166-6083 e-ISSN: 2166-6113
2018; 8(1): 11-17
doi:10.5923/j.fs.20180801.02

Pre-tuber Application of Fluridone: Effect of Foliar and Root Absorption on Sprouting of Yam (Dioscorea alata L.) Tubers
Somina Braide, Elsie I. Hamadina
Department of Crop and Soil Science, Faculty of Agriculture, University of Port Harcourt, Choba, Nigeria
Correspondence to: Elsie I. Hamadina, Department of Crop and Soil Science, Faculty of Agriculture, University of Port Harcourt, Choba, Nigeria.
| Email: |  |
Copyright © 2018 The Author(s). Published by Scientific & Academic Publishing.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The absorption of Fluridone (an abscisic acid biosynthesis inhibitor), prior to yam tuber formation has been shown to induce precocious sprouting on the new tubers that form. However, the use of hydroponics system to achieve root absorption of Fluridone requires high skills and capital, and the effect of pre-tuber application of Fluridone on sprouting of yam tubers produced in a soil medium is not known. Thus, the objectives of this study were to: (1) determine the effect of 30µM Fluridone on sprouting of soil produced yam tubers, and (2) to determine the effect of different methods of applying (root and foliar application) 30µM Fluridone on the timing of sprouting of soil produced yam tubers. The treatments were: (1) nutrient solution medium (Control 1) (2) nutrient solution medium + 30µM Fluridone (root absorbed) (3) soil medium irrigated with distilled water (Control 2) (4) soil medium+ 30µM Fluridone (foliar absorbed) (5) soil medium + 30µM Fluridone (root absorbed). The study was a completely randomized design with five treatments. Results showed that Fluridone treatments induced sprouting on both aerial and new underground tubers while the controls did not. Soil + 30µM Fluridone (foliar absorbed) treatment yielded 36% less sprouting underground tubers but over 50% more sprouting aerial tubers compared to the nutrient solution + 30µM Fluridone treatment. Both treatments however, produced sprouts at 5 weeks after treatment application. Soil + 30µM Fluridone (foliar absorption) treatment induced significantly (P<0.05) more sprouts than soil medium + 30µM Fluridone (root absorption) and sprout was delayed by two weeks in the later. Thus, this study has shown that Soil + 30µM Fluridone (foliar absorption) treatment, which is a cheaper, less skill requiring less cumbersome method, is a potential alternative to the use of nutrient solution + 30µM Fluridone in a hydroponics system for sprout induction.
Keywords: Dioscoreaalata, Dormancy, Fluridone, Food Security, Seed Tubers
Cite this paper: Somina Braide, Elsie I. Hamadina, Pre-tuber Application of Fluridone: Effect of Foliar and Root Absorption on Sprouting of Yam (Dioscorea alata L.) Tubers, Frontiers in Science, Vol. 8 No. 1, 2018, pp. 11-17. doi: 10.5923/j.fs.20180801.02.
Article Outline
1. Introduction
- Dormancy is an adaptive mechanism that imposes a programmed inability on plant parts, however viable, to commence vegetative growth/sprouting owing to factors that are endogenous or exogenous to it [1]. In many plant parts, the induction of dormancy commences during early development such as soon after embryo maturity in some seeds [2-5] or during tuber initiation as in yam (Dioscorea spp.), potato [6, 7]. However, in other cases, dormancy is said to commence at seed or tuber maturity, which in the case of tubers coincides with the onset of the dry season [8, 9]. Thus, the duration of dormancy varies depending on species, treatments given and timing of harvest of the affect part. In yam therefore, this duration ranges from 4-6 months; being too long to allow more than one generations of yam per year.Yam is an important crop in Africa and indeed globally [10, 11, 8, 12]. The food, foreign exchange, socio-economic and industrial value of its tuber has been well discussed. All these values compete for the tubers available during the harvesting season and the competition increasing even more with the onset of the planting season when the tubers are also required as planting material. These competing roles of the tuber together with consistently low supply/availability to tubers lead to high cost of yam production, with the cost of planting material alone accounts for more than 40% of the cost of yam production [13, 14]. Since sprouting/germination is the visible mark of the absence of dormancy (irrespective of whether the dormancy was released, broken or prevented), the development of methods that can induce early sprouting on soil grown seed tubers is an important option for increasing yam tuber annual yield and availability. This option will encourage at least two cycles of yam production per year, reduce the cost of tuber planting material, which will in turn reduce the cost of yam production and encourage the cultivation of larger land area.The inability to induce sprouting on soil grown tubers has caused research to focus on the developing methods that can increase the availability of tubers through the cultivation of other yam parts such the vines. The production of seed tubers from vines has advanced from growing vines on soil medium to growing them in controlled environments using techniques such as aeroponics, hydroponics, Temporary Immersion Bioreactors (TIB) etc [15, 12]. These systems can no doubt produce clean tubers and increase seed tuber availability, but they are very expensive to operate, requires skills and do not remove dormancy from the seed tubers produced. Thus, in studies that require the evaluation of tubers, such precious tubers must be stored until dormancy is released. Unfortunately, storage exposes precious tubers to undesirable conditions such as: unplanned consumption, pests and diseases attack, which lead to reduction in the quantity of tubers available for planting [16, 1]. Using traditional system such as the storage of a fraction of harvested tubers or minisett technology, small and large-scale yam tuber production systems are still faced with the problem of rarity, high cost of planting materials [12] and the tubers produced still express dormancy. Therefore, there is still need to seek for methods that can induce early sprouting on tubers at the will of researchers and farmers.Recent studies have shown that early sprouting is achievable on new tubers when yam plant absorbs Fluridone prior to tuber formation [1, 17]. This has been demonstrated in vitro using plantlets derived from apical meristems [1, 17] as well as in a hydroponics system using plants derived from minisetts [18]. In these cases, new tubers were initiated and induced to sprout less than 40 days after the commencement of the treatment. Compared to natural sprouting which occurs not less than 270 days after tuber initiation, the results obtained in those studies are phenomenal. Fluridone is an herbicide that is famed for its ability to inhibit ABA biosynthesis through the inhibition of the production of carotene, which is the precursor of the plant hormone ABA [19, 20]. Nevertheless, the methods are expensive and require skills. Moreover, there are no known methods to induce sprouting on seed tubers developing in soil medium. Thus, this study seeks to answer the following questions: can seed yam tubers developing in soil medium be induced to sprout during early tuber development using Fluridone very much as in vitro and hydroponically grown tubers? Would other cheaper and easier methods of applying Fluridone (such as via irrigation or foliar) induce sprouting on tubers developing in soil medium? Must Fluridone be absorbed via roots to be effective? The objectives of this study were therefore to: (1) determine the effect of 30µM Fluridone on sprouting of soil produced yam tubers, and (2) to determine the effect of different methods of applying (root and foliar application) 30µM Fluridone on the timing of sprouting of soil produced yam tubers.
2. Materials and Method
2.1. Study Environment
- This study was conducted in the Department of Crop and Soil Science screen house, Faculty of Agriculture, University of Port Harcourt. The screen house was constructed with plastic mesh sheet and transparent plastic roofing sheets. Thus, the screen house allows for ventilation. Because there is a proportional relationship between the number of photons absorbed in 400 to 700 nm band and the rate of photosynthesis in plants, the photosynthetic active radiation (PAR) level in and outside the screen house was monitored using a quantum PAR meter (Hydro Farm product). Temperature and relative humidity in and outside the screen house were taken using a temperature and relative humidity sensor, KT908.
2.2. Planting Material
- Tubers of Dioscorea multiplied in preparation for this study. The tubers used in this study were harvested in December 2016 and stored until required for this study. This variety was chosen because it is known to exhibit long tuber dormancy and presents a potential commercial value as an alternative source of various dietary requirements as well as research material for various purposes thereby relieving the more accepted variety, D. rotundata of some pressure and increasing the acceptability of D. alata.
2.3. Preparation of Minisetts
- Minisetts were obtained from the proximal region of the non-dormant tubers. The tuber-head, which is the corm-like structure attached to the proximal region of the tuber [21, 22] was severed from the tuber (where present) to inhibit apical dominance consequently redirecting growth/shoot emergence to other parts. Minisetts weighing approximately 50g were obtained by longitudinally cutting through them making sure that every minisett had a measure of the head region because the head region of tubers tend to sprout faster than the middle or distal regions [23]. The cut ends of all the minisetts were treated by dabbing them in wood ash to minimize rotting [24].Treated minisetts were air-dried for 24h then planted in baskets filled with moist sawdust. The base and sides of the baskets were lined with 2mm net to prevent loss of the sawdust through the holes. Daily observation of the minisetts for vine emergence was done. Watering was done when necessary. At 10 days after pre-sprouting, most of the minisetts had initiated sprouts.
2.4. Rooting of Pre-sprouted Minisetts
- Sprouted minisetts were planted to poly-pots containing soil and well cured poultry manure mix in the ratio 25:1. Watering was done when necessary. The minisetts were grown in the poly pots to root.
2.5. Transplanting of Rooted Plants to Treatment Pots
- Soil on individual plants was shaken off to expose the roots and then washed before transplanting them randomly into plastic pots (5 inches) containing 100ml distilled water or moist soil. The pots were cut 5 inches above the base and perforated – the pots for the hydroponics treatment were perforated in sets of threes along the side of the pots with the first perforation done 1.0 inch above the base. Those for the soil treatments were perforated in a similar manner but with more perforations at the base of the pots. The pots were previously disinfected and thoroughly rinsed before use. Disinfected (in 5% sodium hypochlorite) coco coir was used to support for the plants and to keep new tubers (when they are initiated) away from light while the roots were placed at the base of the pot.The soil pots were filled with 5kg of soil that was collected from around the Department of Crop and Soil Science, University Port Harcourt and then air-dried to constant weight. The soil was then brought to field capacity two days before the start of the study. The application of 100 ml of test solution kept the soil at field capacity. A soil moisture probe instrument was used to monitor soil moisture levels. Soil samples were collected prior to and at the end of the experiment for nutrient analysis.
2.6. Solutions and Reagents
- The Hoagland nutrient solution was used for the hydroponics treatments throughout the study. Fluridone was purchased from ChemServices, USA.
2.7. Experimental Treatments and Application
- Treatment commenced two days after transplanting. The experiment consists of five treatments: 1. Nutrient solution medium (Control 1), 2. Nutrient solution medium + 30µM Fluridone (root application), 3. Soil medium irrigated with water (Control 2), 4. Soil medium + 30µM Fluridone (foliar application by spraying) and, 5. Soil medium + 30µm Fluridone (root application by soil irrigation).All treatments were applied at a rate of 100 ml per plant. Treatments were applied every two days until three cycles were achieved. In treatment 4, the test solution was sprayed on plant leaves while ensuring that the solution did not drip to the soil; the soil was covered with a black polyethylene film during spraying. Spraying was done at 40psi using a cone shaped nozzle attached to a hand sprayer. At the end of treatment applications, the plants in treatment 2, continued to grow in nutrient solution for seven weeks while those in treatments 4 and 5 continued to grow in soil watered with distilled water.
2.8. Experimental Design
- The experiment was designed as a Completely Randomised Design (CRD) with five treatments, six plants per treatment per replicate and three replicates. The pots were arranged on benches and treatments were randomly assigned (Plate 1). These treatments and design are adequate to answer the research questions.
 | Plate 1. Layout of pots after transplanting |
2.9. Data Collection
2.9.1. Number of Sprouts and Tubers Per Sprouting Plant
- The date of appearance of sprout(s) on newly initiated tuber(s) was recorded. Number of sprouts present and number of tubers per sprouting plant was also noted. This was done at 3, 5, 7 and 13 weeks after treatment (WAT), and the data was used to determine the number of sprouts produced and number of sprouts per tuber.
2.9.2. Leaf Chlorophyll Content
- In situ leaf chlorophyll content was determined of done at 4, 5 and 8 WAT using a hand-held chlorophyll meter called at LEAF CHL STD. The chlorophyll meter compares the transmission of light in red and near infrared wavelengths to give a measure of chlorophyll content in green leaves. Chlorophyll values above 35 suggest that the plant is healthy. Two readings per leaf were taken on four representative leaves per plant and two plants per treatment per rep.
2.9.3. Determination of Dry Matter Content
- At the end of the study, two plants per treatment/rep were sampled for this analysis. They were separated into their component parts, dried in a forced air oven at 80°C and dry weights recorded. Number of tubers per plant was also recorded.
2.9.4. Growth Data and Aerial Tubers
- Growth data was collection at 2, 3, 4, and 5 WAT on two pre tagged plants per treatment per replicate. Leaf length and width were repeatedly measured on six leaves per plant. Number of leaves per plant was counted. The number of aerial tubers and number of sprouts on the aerial tubers were counted.
2.10. Data Analysis
- Data analysis was run on GENSTAT 12th Edition software using one-way ANOVA program. All count data were transformed using square root transformation prior to data analysis. Where such count data ranged from 0 to greater than one, one was added to all numbers prior to square root transformation. Means were separated using standard error of difference (s.e.d) at 5% probability level.
3. Results and Discussion
3.1. Environmental Conditions
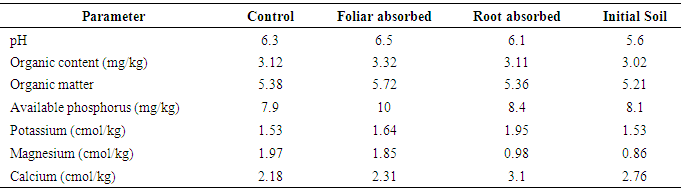
- The average temperature and relative humidity inside and outside the screen-house varied only slightly. The average temperature at 7am, 2pm and 6pm in the screen-house was 22.8, 29.2, and 26.5°C respectively while average relative humidity at the same times were 82.7, 76.3 and 82.6% respectively. The average photosynthetic active radiation (PAR) reading at lower and upper plant levels was 88 and 124 µmoles/m2/s respectively.Results from soil physical analysis shows that the soil used in this study was sandy loam (77.6% sand, 10.7% silt and 13.3% clay) and this composition did not change following treatment application. The pre- and post-treatment chemical characteristics of the soil used in this study are shown in Table 1.
|
3.2. Effect of Treatments on Morphology
- Bleaching of leaves was observed at two weeks after treatment in the Soil medium + 30µM Fluridone (foliar application by spraying) treatment and three weeks after treatment application in Nutrient solution medium + 30µM Fluridone (root application). In the soil medium + 30µM Fluridone (root application by soil irrigation) treatment, bleaching of leaves commenced much later; at 7 WAT. Some leaves showed extensive bleaching with a purplish and/or white stem/vine. Bleaching was complete in some leaves and patchy in others. Some leaves of fluridone treated plants were longer than usual. These observations suggest that there is some link between the channel (root or foliar) of Fluridone absorption, growing medium and the intensity of whitening inducible by fluridone.
3.3. Effect of Treatment on Sprouting
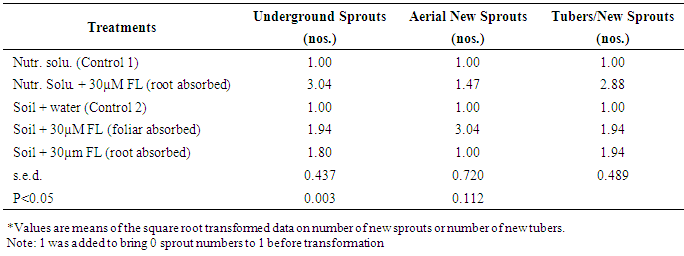
- There were no new sprouts on underground or aerial tubers produced in the Control treatments. Sprouts were first observed above the surface of the cococoir at 34 d after treatment (i.e., 5 WAT) and this was found to emerge from the new underground tubers that developed in Fluridone treatment (i.e., Nutr. Solu. + 30µM FL root absorbed and Soil + 30µM FL foliar absorbed). Sprouts were observed two weeks later (i.e., at 48 d after treatment or 7 WAT) in the Soil + 30µM FL irrigated treatment. These observations further strengthen the suggestion that there is some link between the channel (root or foliar) of Fluridone absorption, growing medium and the intensity of whitening inducible by fluridone.By the end of the study, the highest number of sprouts was observed in the Nutr. Solu. + 30µM FL (root absorbed) and this was significantly (P<0.05) higher than the number of sprouts in either Soil + 30µM FL foliar absorbed or Soil + 30µM FL root absorbed treatments (Table 2). Although the number of sprouts produced in the Soil + 30µM FL (root absorbed) treatment was fewer than that produced in the Soil + 30µM FL (foliar absorbed) treatment, the difference was not significant at P<0.05.Compared to the Control, the Soil + 30µM FL (foliar absorbed) and Soil + 30µM FL (root absorbed) treatments produced 36% and 59% less sprouts respectively. This suggests that Fluridone application (whether foliar or irrigated) can induce early sprouting on underground tubers produced in a soil medium. It suggests that Soil + 30µM FL (foliar absorbed) can be a suitable alternative to Nutr. Solu. + 30µM FL (root absorbed) treatment, and that the soil system might hamper the uptake of fluridone. The number of new tubers per new sprout followed the same trend as the number of new sprouts on underground tubers (Table 2). In contrast, however, foliar application of fluridone yielded the largest number of new sprouts on aerial tubers and this was significantly (P<0.05) higher than the number of new sprouts on Nutr. Solu. + 30µM FL root appl (Table 2). The Soil + 30µM FL (root absorbed) treatment did not yield any sprouting aerial tubers and this was similar to that in the Controls. Thus, Soil + 30µM FL (foliar absorbed) can be a suitable alternative to Nutr. Solu. + 30µM FL (root absorbed) treatment since it does not only produce reasonably good number of sprouting underground tubers but also large numbers of sprouting aerial tubers with no direct contact on soil properties.
|
3.4. Effect of Treatment on Vegetative Growth
3.4.1. Leaf Length and Width and Number of Leaves
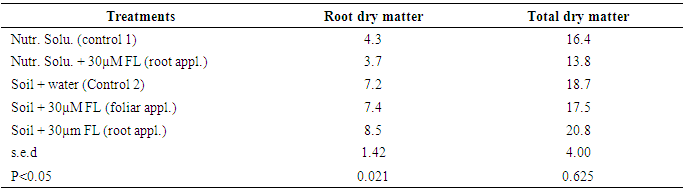
- The treatments in this study had no significant effect on leaf length and width between the 2nd and 5th week after treatment. Also, treatments had no significant effect on number of leaves at 2, 3, 4 and 5 WAT. Dry matter contentThe analysis of dry matter content conducted at the end of the study (at 8 WAT) shows that stem (petiole inclusive), leaves and tuber dry matter content did not vary significantly across treatments. However, there was significant difference in root dry matter content across treatments (Table 3). The difference was mainly between plants growing in the soil system and those growing in nutrient solution with root dry matter being significantly higher in all treatments in the soil system than those in nutrient solution. Treatments within the soil system did not vary significantly suggesting that fluridone did not negatively affect root dry matter content. Variation in total dry matter content followed the same trend as that of root dry matter content.
|
3.4.2. Number of Tubers Produced
- Analysis of the number of tubers produced per treatment at the end of the study (at 8 WAT) shows that plants produced 1 to 6 underground tubers per plant, and there was no significant difference across treatments in number of tubers.
4. Discussion
- This study sought to evaluate the potential of Fluridone to cause underground seed yam tubers to sprout during early tuber development in a soil medium and to assess the effect of cheaper, less skill requiring methods of applying Fluridone on sprouting. In this study, the newly initiated tubers in soil medium were observed to have sprouts/visible shoot buds on their head region irrespective of whether Fluridone was applied to the plants as foliar spray or via soil irrigation. This ability of Fluridone to induce sprouting on micro yam tubers soon after its initiation has been demonstrated in vitro using plantlets derived from apical meristem [1, 17] and in a hydroponics system using plants derived from minisetts [18]. Also, working on other plant species, Fluridone has been shown to promote germination/sprouting on dormant [25, 26, 27] and tubers such as potato [6, 7]. What this study has shown is that Fluridone can induce early sprouting on underground yam tubers that are developing in a soil medium. Thus, although in vitro and hydroponics systems ensure that Fluridone is available to the bare roots for absorptions, yam plants can also absorb Fluridone via leaves and via the complicated soil system.A comparison of the effects of the different treatment methods on sprouting of underground tubers showed the following order: Nutr. Solu. + 30µM FL (root absorbed) > Soil + 30µM FL (foliar absorbed) > Soil + 30µM FL (root absorbed), while the control treatments did not induce any sprouts. The fact that Soil + 30µM FL (foliar absorbed) treatment produced only 36% less sprouts compared to the Nutr. Solu. + 30µM FL (root absorbed) method, which requires high skills and capital intensive, makes it a suitable alternative method for inducing sprouting on young underground tubers. Other added advantages of the Soil + 30µM FL (foliar absorbed) method are that the soil ecosystem is protected since the soil is covered during application, it induces the production of twice as much sprouting aerial tubers compared to Nutr. Solu. + 30µM FL (root absorbed) method, it does not have a significant negative effect on vegetative growth and tuber dry weight, and it significantly increases root and total dry weight compared to Nutr. Solu. + 30µM FL (root absorbed) method. The ability of Fluridone to cause increase in root dry weight has been reported severally [17, 28, 29]. Thus, the potential for field application of this method is high.The application of Fluridone by via soil irrigation water led to the production of sprouting underground tuber but no sprouting aerial tubers. Visible evidence of Fluridone uptake (whitening of leaves) was delayed by up to two weeks in using this method and the effect was mild. It is perhaps this delay that contributed to the production of almost 60% less sprouting underground tubers compared to the Nutr. Solu. + 30µM FL (root absorbed) method. The delay in the expression of a fluridone effect may connect with the low mobility of Fluridone in soils (owing to its Koc value of 350 to 2,462) and its high adsorption (pKa values of 12.3) tendency to soil particles, which may have in turn limited its availability to yam roots. Although Soil + 30µM FL (foliar absorbed) method did not lead to the production of significantly (P<0.05) more sprouting underground tubers compared to Soil + 30µM FL (root absorbed) method, it is apparently superior to the Soil + 30µM FL (root absorbed) method. Fluridone is easily degraded (within 1-6 days) by light (NCBI, 2018) however, its persistence in sandy loam soil is controversial (NCBI, 2018). Residual negative effect on maize growth was observed when maize was grown about 30 days after harvesting of a yam crop, which grew for 13 weeks on soil treated Fluridone [27]. Indeed, Fluridone effect was still observed on maize after prolonged storage of the soil in conditions that exposed it to several cycles of wetting and drying; to simulate the raining season effect, and then to prolonged dryness over the dry season in the humid southern Nigeria [30]. Although Fluridone is not known to negatively affect animal health at low concentration per day persistence of Fluridone in soils must be curtailed.
5. Conclusions
- In conclusion new yam tubers are induced to produce sprouts on their surface if Fluridone is applied to the yam plants (before the new tubers are initiated) either via foliar spraying while the soil is covered or via irrigation water to soils holding the yam plants. These methods require almost no skills and are far easier and cheaper to operate than the use of the hydroponics method. Nevertheless, the foliar spray method is more environmentally, economically and socially friendly method than the soil application method. Thus, it is a potential alternative to the hydroponics methods. This finding has commercial value in the production of seed yam tubers and to researchers who do not want their activities to be restricted by the phenomenon of dormancy.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML