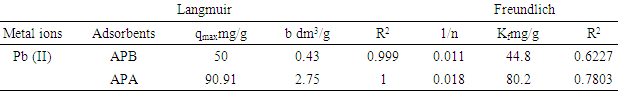-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Frontiers in Science
p-ISSN: 2166-6083 e-ISSN: 2166-6113
2018; 8(1): 1-10
doi:10.5923/j.fs.20180801.01

Aluminophosphates Derived from Tea Leaves and Pumpkin Seeds Ashes Adsorb Lead Ions from Contaminated Water
F. M. Maingi, H. M. Mbuvi, M. M. Ng’ang’a
Department of Chemistry, Kenyatta University, Nairobi, Kenya
Correspondence to: H. M. Mbuvi, Department of Chemistry, Kenyatta University, Nairobi, Kenya.
| Email: |  |
Copyright © 2018 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

One major global challenge is the existing disparity in water distribution resulting to millions of people lacking access to safe clean water. This will certainly be exacerbated by the impending climate change and increased pollution threats posed by industrialization and population growth. Therefore pursuing sustainable materials and technologies for water remediation is crucial. Adsorbents use for water treatment is preferable owing to its simplicity and cheap materials involved. Aluminophosphates molecular sieves have open-framework structures that possess a wide range of pore openings and large surface area for use as adsorbents that can be synthesized hydrothermally using alumina and phosphates. Literature reports indicate that accumulation of aluminum in tea leaves correlates positively with leaf age. The EDXRF analysis showed that tea leaves ash used contained 30.09% of Al2O3. Similarly, literature findings indicate that pumpkin seeds contain relatively large amounts of phosphorous with the pumpkin seed ash used containing 37.01% of P2O5. Consequently, aluminophosphate APA and APB were synthesized using tea leaves to pumpkin seeds ash ratios of 1:1 and 1:2 respectively. XRD characterization and lead ions batch sorption experiments were done. Turbidity column experiment was done. Data for adsorption of Pb (II) ions data on the adsorbents best fitted into the Langmuir isotherm model. Adsorption capacities obtained from the linearized graphs were 90.91 and 50.0 mg/g for APA and APB respectively. This shows that tea leaves and pumpkin waste can synthesize aluminophosphate adsorbents. Aluminophosphates have shown good potential of removing Pb (II) ions from wastewaters.
Keywords: Adsorption isotherms, Heavy metals, Aluminophosphates, Wastewaters, Adsorbents
Cite this paper: F. M. Maingi, H. M. Mbuvi, M. M. Ng’ang’a, Aluminophosphates Derived from Tea Leaves and Pumpkin Seeds Ashes Adsorb Lead Ions from Contaminated Water, Frontiers in Science, Vol. 8 No. 1, 2018, pp. 1-10. doi: 10.5923/j.fs.20180801.01.
Article Outline
1. Introduction
- Access to safe clean water remains a major global challenge of our time. Current reports indicate that 663 million people have no access to clean drinking water while 2.4 billion people still lack proper sanitation facilities [1]. Inadequate access to clean and safe water is experienced in many regions of sub-Saharan Africa and severe disparity in its distribution exists. This has led to increased cost of water cost and water purification technologies due to high demand. Further, the little available clean water is threatened by pollution from domestic and industrial waste. The projected increase in population and industrial growth will likely exacerbate the pollution situation. Therefore, pursuance of sustainable materials and technologies for water treatment is essential. Among the methods currently in use, adsorption allows use of cheap renewable and sustainable materials and offers simplicity that includes developing customized household water treatment kits. Aluminophosphate molecular sieves, which can be cheaply, synthesized hydrothermally using alumina and phosphates contain open-framework structures with a wide range of pore openings and large surface area for use as adsorbents [2]. Literature reports indicate that tea plant is a fluoride (F) and aluminum (Al) hyper accumulator [3]. Several works have established that the concentration of Al in tea leaves positively correlate with the age of the leaves, with older leaves accumulating about 10 times more than young leaves [4]. Concentrations of between 5000– 30 000 μg·g–1 have been reported in old leaves. Similarly, literature findings indicate that pumpkin seeds contains relatively large amount of phosphorous. Indeed, dry weight concentration levels of 15.7 mg/g have been reported [5]. According to Suphakarn, 100 g of roasted pumpkin contains about 955 mg of phosphorous [6]. Suitably, the present study aimed to synthesize aluminophosphate molecular sieves using tea leaves ash, TLA and pumpkin seeds ash, PSA as sources of alumina and phosphates respectively. This article reports the synthesis of two adsorbents from TLA and PSA, their characterization as well as their adsorption efficiencies and capacities for lead ions from contaminated water.
2. Materials and Methods
2.1. Chemicals
- Chemicals and solvents of analytical grade were used without further purification. A stock solution containing 1000 mg/L of lead was prepared by dissolving 1.559 g of lead (II) nitrate in 1000 mL of distilled water in volumetric flask. This stock solution was then diluted to obtain working solution containing 100 mg/L and standard solutions of 0, 2, 4, 6, and 8 mg/L. the pH of the solutions were adjusted to acidic and basic conditions using nitric acid and sodium carbonate solutions respectively [7].
2.2. TLA and PSA Preparation
- Tea leaves obtained from farms in Muranga were transported to the chemistry laboratory at Kenyatta University where they were extensively washed with tap water to remove dust, rinsed with distilled water and then air dried. Ashing was done using an oven at a temperature of 400°C for three hours. The resultant ash was mixed to obtain a composite sample then sieved to obtain particles of the same size. Pumpkin seeds from vendors in Molo were also transported to the chemistry laboratory where they were washed several times with distilled water to remove soil and dust and then dried at 100°C. The clean and dry pumpkin seeds were burnt in an oven at 800°C for 6 hours to obtain ash. The ash was mixed and sieved to obtain fine particles of the same size.
2.3. Synthesis of Aluminophosphate Material A (APA)
- About 30 g of TLA was thoroughly mixed with about 30 g of PSA. This ash was placed in a 1000 mL glass beaker, 400 mL of distilled water added to it and then stirred continuously. The resultant mixture was transferred into a cylindrical stainless steel metallic reactor and 100 mL of a 28% ammonia solution added to it. The reactor was closed airtight and introduced into a pre-heated oven at 100°C for a period of 24 hrs. After which the contents were allowed to cool then filtered. The solid residue was washed with distilled water to remove the excess ammonia solution and dried at 100°C for 12 hrs. This solid residue was designated as APA.
2.4. Preparation of Aluminophosphate Material B (APB)
- For the preparation of APB, approximately 20 and 40 g of TLA and PSA respectively were put into the metallic reactor, 400 mL of distilled water followed by 100 mL of 28% ammonia solution were added and then the mixture stirred. The reactor was placed in a preheated oven at 100°C for 24 hrs. The resultant solid was washed with distilled water to remove the excess ammonia then dried at 100°C for 12 hrs. The samples were designated as APB.
2.5. Batch Experiments
- A temperature-controlled water-bath shaker (DKZ-1 NO.1007827) was used for the batch adsorption experiments. The experiments were performed at the same shaking speed. For each experimental run, 50 mL aqueous solution of known concentrations of Pb (II) ions were put in 100 mL plastic bottles that contained known masses of APA and APB. These bottles were agitated at a constant shaking rate of 420 rpm and temperature of 25°C for the specified period (time) after which the contents were filtered. The concentrations of Pb(II) ions in the resulting filtrates were determined using a flame atomic spectrometer. Amount of Pb (II)ions adsorbed per unit mass of adsorbent and the percentage of metal ions removed were calculated using equations 1 and 2 respectively.
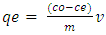 | (1) |
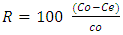 | (2) |
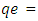 Amount of Pb (II) ions adsorbed per unit mass of adsorbent at equilibrium
Amount of Pb (II) ions adsorbed per unit mass of adsorbent at equilibrium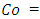 Initial concentration of sorbate
Initial concentration of sorbate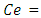 Concentration of sorbate at equilibrium
Concentration of sorbate at equilibrium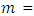 mass of sorbate (g) (atomic mass)
mass of sorbate (g) (atomic mass) 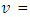 volume of solution, (L)
volume of solution, (L)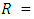 percentage of Pb (II) ions removed from the 50 mL aqueous solution
percentage of Pb (II) ions removed from the 50 mL aqueous solution2.6. Optimization of the Various Parameters on the Percentage of Pb (II) Ions Adsorbed
- The effects of various parameters (pH, adsorbent dose, contact time, initial concentrations, shaking speed and temperature) on removal efficiencies were probed by determining changes in the percentage of Pb (II) ions adsorbed as the parameters of interest were varied while keeping the others constant.
2.6.1. Optimization of pH
- The effect of pH was probed by determining changes in the percentage removal of lead from 50 mL of 100 mg/L Pb (II)ion solutions that were agitated at 420 rpm and maintained at 25°C for a contact time 60 minutes using 0.1 g of adsorbents as pH was varied from 2 to 6. The pH adjustments were done using Na2CO3 and HNO3 acid.
2.6.2. Optimization of Time
- The effect of contact time was investigated by varying contact time from 20 to 100 min at same conditions of: 0.1 g of adsorbents, 100 mg/L Pb (II) ion solutions for APA and APB pH of 5.0, temperature of 25°C and shaking speed of 420 rpm.
2.6.3. Optimization of Shaking Speed
- The shaking speed was investigated by varying shaking speed from 120 to 600 rpm at same conditions of: 0.1g of adsorbents, 100 mg/L Pb (II) ion solutions for APA and APB, temperature of 25°C, shaking time of 60 minutes and pH of 5.0.
2.6.4. Optimization of Temperature
- The effect of temperature was investigated by varying the temperature from 20 to 100°C at same conditions of: 0.1g of adsorbents, 100 mg/L Pb (II) ion solutions for APA and APB and, agitation speed of 420 rpm, pH of 5.0 and contact time of 60 minutes.
2.6.5. Optimization of Sorbent Dose
- The effect of the sorbent dose was investigated by varying the doses from 0.1 to 0.5g at same conditions of 100 mg/L Pb (II) ion solutions for APA and APB, pH of 5.0, shaking speed of speed of 420 rpm, temperature of 25°C and contact time of 60 minutes.
2.6.6. Effect of Initial Metal Ion Concentration
- The effect of initial concentration was investigated by varying initial concentration from 20 to 500 mg/L at same conditions of: 0.1 g of adsorbents, temperature of 25°C, shaking speed of 420 rpm, pH of 5.0 and contact time of 60 minutes.
2.7. Removal of Turbidity from Water
- Studies on the percentage removal of turbidity by the adsorbents were conducted in a glass column made of Pyrex glass tube of (0.6m) height and (0.025m) inner diameter. Cotton wool was attached at the bottom of the column followed by a layer of glass beads. various quantities (0.1, 0.2, 0.3, 0.4 and 0.5 g) of the adsorbents were packed in the column and then cotton wool inserted on top of the bed and firmly secured in place by layer of glass beads in order to provide a uniform flow of the solution through the column. Once the adsorbents had been packed inside the column, distilled water was passed through the column to ‘wet’ it. This was important to ensure that all air was expelled between and within the aluminophosphate particles before the experiment began. If there was an air pocket inside the column, channeling and air entrapment would occur which would lower the bed performance. A 50 mL volume of turbid water of known turbidity (206.75 NTU) was then made to flow through the column, collected from the outlet of the column and its turbidity measured.
3. Results and Discussions
3.1. Samples Characterization
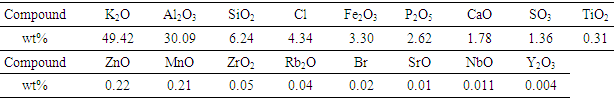
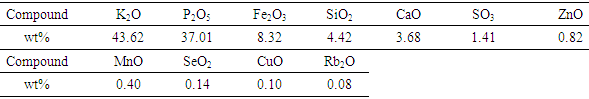
- The chemical composition of TLA and PSA was determined using EDX spectroscopy and the results are shown in tables 1 and 2 respectively. As shown in table 1, the major elements/oxides found in TLA were K2O, Al2O3, SiO2, Cl, Fe2O3, P2O5, CaO and SO3 approximated at percentages of 49.4, 30.1, 6.2, 4.3, 3.3, 2.6, 1.8 and 1.4 respectively. This confirms that it is a good source of alumina. The amount of aluminium oxide in old tea leaves is in agreement with the data reported by Dong et al. [8].
|
|
3.2. Characterization of Aluminophosphate Materials APA and APB
- The X-ray diffraction pattern of APA and APB is presented in Figure 1 and 2 respectively. The diffractograms of APA (synthesized using 1:1 TLA to PSA mass ratio) confirmed the presence of Kaolinite (20.9%), smectite (6.7%), AlPO-8 (34.1%), quartz (12.6%), ilitite (2.3%) and amorphous (23.4%) [9]. In comparison the sample APB treated with ratio of TLA to PSA of 1:2 also revealed different peak intensity. Peaks in the diffractograms confirmed presence of kaolinite (15.6%), smectite (4.4%), AlPO-H2 (28.7%), ilitite (2.2%), Quartz (20.1%) and AlPO-C (5.2%). This implies the importance of ash ratios as the main contributory factor for differences in chemical composition of APA and APB.
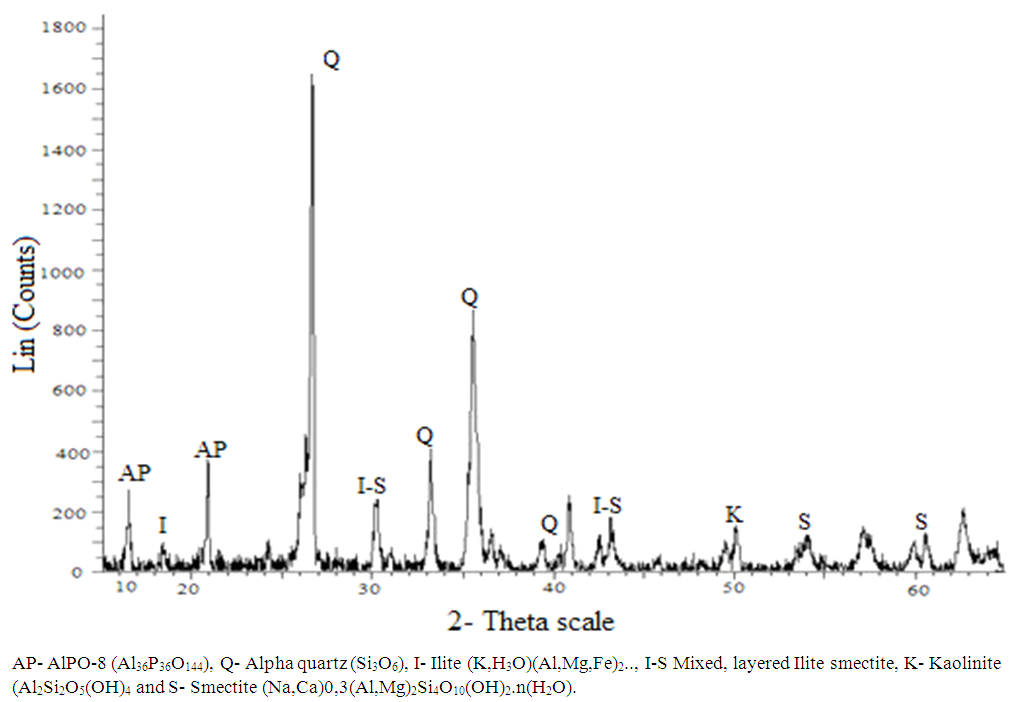 | Figure 1. Powder XRD diffraction pattern of APA |
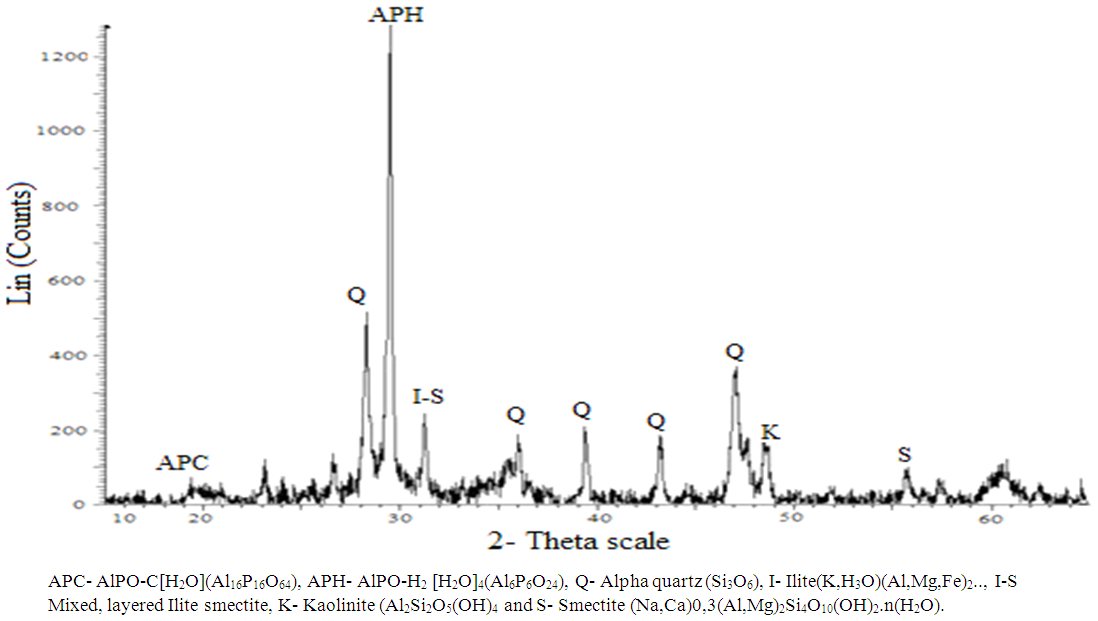 | Figure 2. Powder XRD diffraction pattern of APB |
3.3. Effects of pH on Percentage Removal of Lead Ion
- The effect of pH was investigated by determining removal efficiency of lead ions as pH was variedfrom 2 to 6. The percentage removal was found to be 76.5% for APA at pH 2, 77.1% for APB at pH 2. Both APA and APB achieveoptimum adsorption at a pH of 5 as shown in figure 3. At low pH, surface of adsorbents are protonated, thus there is repulsion between the surface of adsorbent and lead ions decreasing the rate of adsorption. As pH increases active sites are less protonated increasing the attraction between the adsorption sites and lead ions. Thereafter, the percentage removal of lead ions remains constant with increase in pH. This is as a result of exhaustion of active sites. Similar results were reported by Sukru et al. [9].
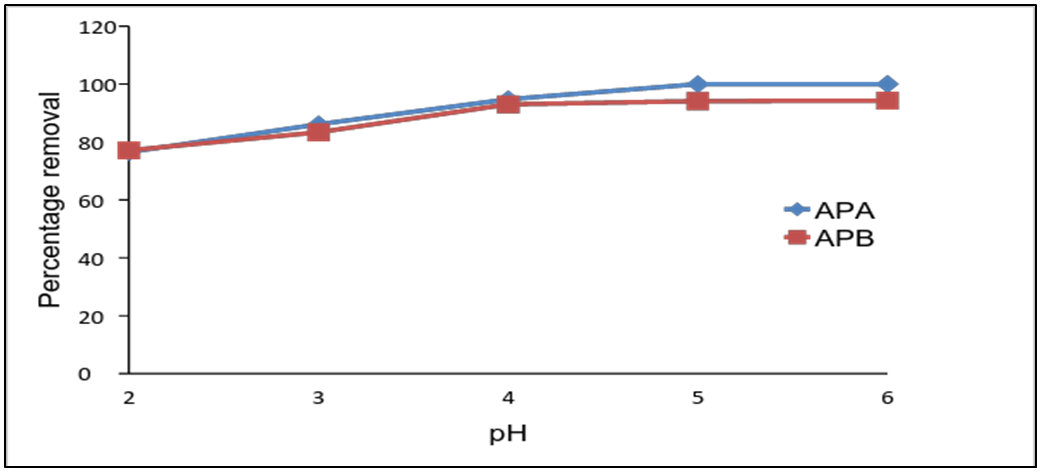 | Figure 3. Effect of pH on the removal of Pb (II) ions by APA and APB (initial solution concentration = 100 mg/L, sorbent dose = 0.1g, agitation speed = 420rpm and temp=25°C) |
3.4. Effect of Contact Time on Percentage Removal of Lead Ion
- Experiments were conducted by varying contact time from 20 to 100 min while maintaining all other experimental conditions constant. The results obtained are shown in Fig.4. As shown the adsorption process occurred rapidly when APA and APB were used with over 96% of the lead present adsorbed by APA and over 88% for APB after 20min.There was an increase rate of adsorption with increase in contact time. APA achieved an optimum of about 99.4% removal after 40 min while APB optimized at about 91% within 80min. Increase in contact time increases the chances of interaction between adsorbate ions and adsorbent sites increasing the rate of adsorption. Equilibrium is attained after exhaustion of the active sites present in the adsorbents. Similar trendshave been reportedin literature [10].
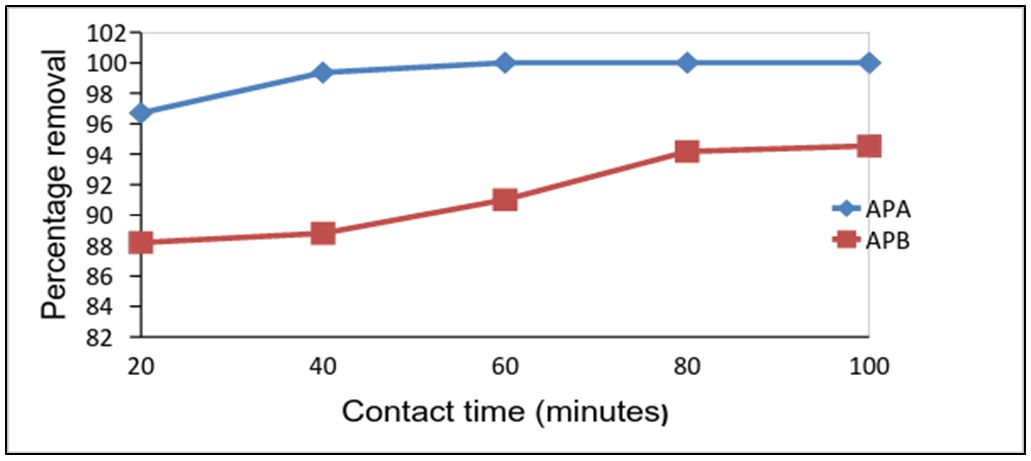 | Figure 4. Effect of contact time on the removal of Pb (II) ions by APA and APB (initial solution concentration = 100 mg/L, adsorbent dose = 0.1g, shaking speed = 420rpm, pH= 5.0 and temp = 25°C) |
3.5. Effect of Shaking Speed on Percentage Removal of Lead Ion
- The effect of shaking speed on the removal efficiency of lead ions is studied as speeds were varied from 120 to 600 rpm. The percentage removal was found to be 99.18% for APA at 120rpm, 88.94% for APB at shaking speed of 120 rpm. APA achieves maximum adsorption at a shaking speed of 480rpm the same for APB as shown in figure 5. At low shaking speed, the adsorbent accumulates and various active sites are buried under the above layer reducing the rate of adsorption. Increase in the shaking speed improves the diffusion of metal ions towards the surface of the adsorbent. It also helps in spreading the sorbent surface making the active sites available for adsorption and this increases the rate of adsorption.
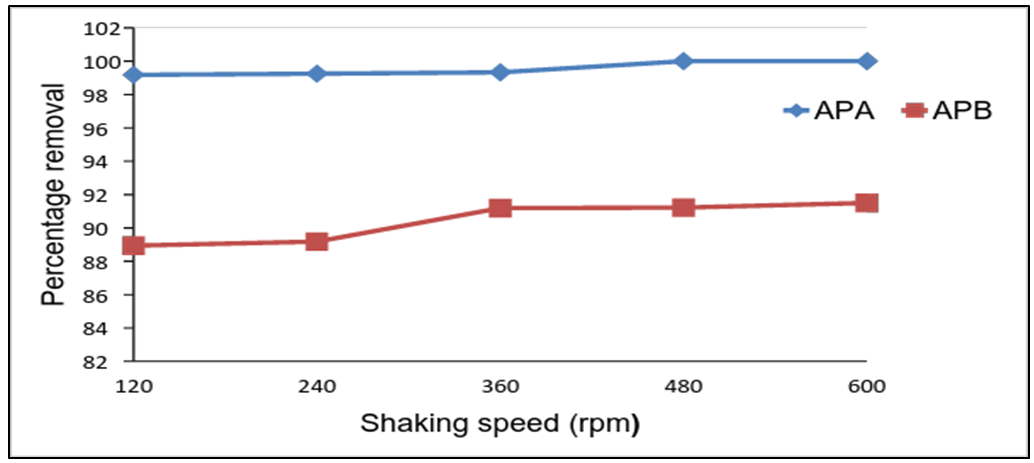 | Figure 5. Effect of shaking speed on the removal of Pb (II) ions by APA and APB (initial solution concentration = 100 mg/L, adsorbent dose = 0.1g, pH= 5.0 and temp = 25°C) |
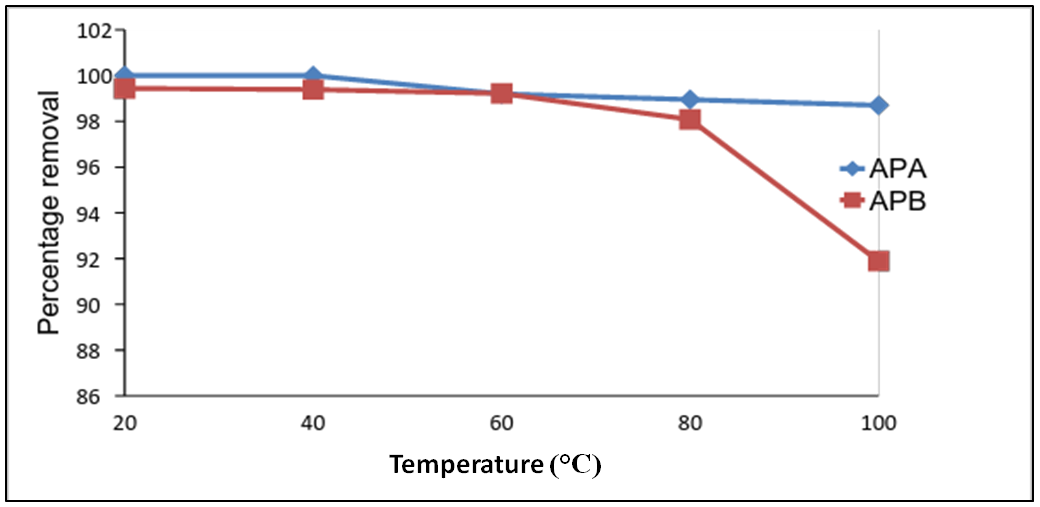
3.6. Effects of Temperature on Adsorption of Lead Ions
- The effect of temperature on the removal of Pb (II) ions by APA and APB from solutions at initial lead ions concentration of 100 mg/L using 0.1 g and contact time of 60 min and agitation speed of 420 rpm as temperatures were varied from 20 to 100°C are shown in figure 6. From the results, it was observed that the percentage removal of lead ions by APA and APB remained fairly same between temperatures of 20 and 60°C at about 99%. The percentage removal of lead ion by APA and APB decreased 98 and 92% when the temperature was increased to 100°C. The observed initial decrease in lead removal with increasing temperature suggests weak binding interaction between the active sites and lead ions which support physiosorption which are exothermic and therefore favoured by low temperature. These results seem to indicate that a high temperature works against the removal of Pb (II) ions [11].
3.7. Effect of Sorbent Dose on Adsorption of Lead Ions
- Batch experiments were conducted using adsorbent doses of 0.1, 0.2, 0.3, 0.4 and 0.5g per 50mL of test solution. When the addition of the adsorbent dose increased, the percentage removal of metal ions also increased as shown in fig 7. Adsorption of lead ions increased due to increase in number of binding sites as the adsorbent dose increased. A maximum removal of 100% at 0.3g APA and 100% at 0.3 g APB was observed. A further increase in adsorbent dose did not have any significant effect on the removal of lead ions from the solution because the adsorbate ions are already exhausted. Similar findings were reported for heavy metal removal from aqueous solutions by using palygorskite clay as an adsorbent [12].
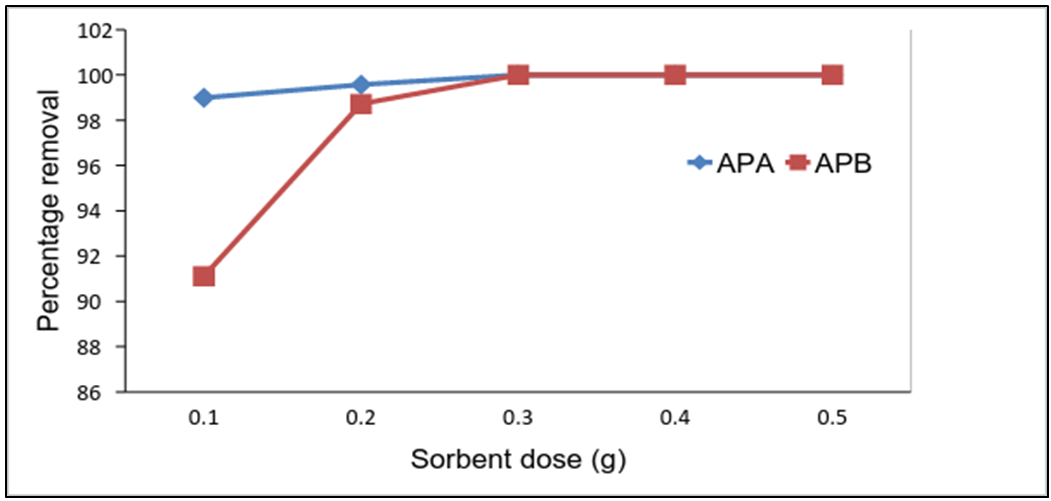 | Figure 7. Effect of dose on the removal of Pb (II) ions by APA and APB (initial solution concentration = 100 mg/L, contact time = 60 min, shaking speed = 420rpm, pH = 5.0 and Temp = 25°C) |
3.8. Effect of the Initial Concentrations of Lead Ions
- The percentage of Pb (II)ions adsorbed by APA and APB was significantly influenced by its initial concentration in aqueous solutions. The initial concentration was varied from 20 mg/L to 500 mg/L while maintaining the pH at 5.0, adsorbent dosage at 0.1g contact time at 60 minutes and adsorption temperature at 25°C. Figure 8 shows the effect of initial concentration on percentage removal of Pb (II)ions. The percentage removal decreased from almost 100% to 35.6 and 19.7% for APA and APB respectively. Increase in concentration lead to a decrease in percentage removal of Pb (II)ions due to an increase in the number of metal ions for the fixed amount of adsorbent. Reddy et al. [] also reported a similar trend for the case of removing Pb (II) ions by Moringaoleifera bark [13].
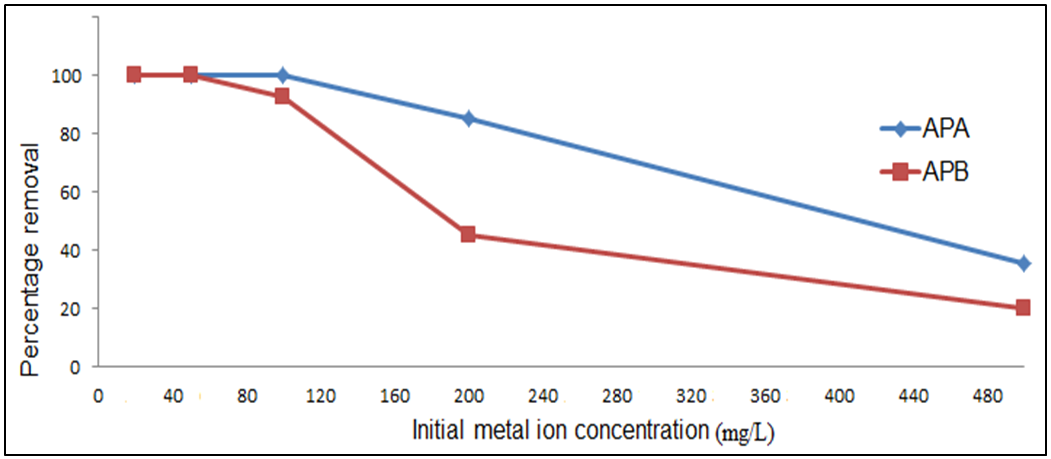 | Figure 8. Effect of initial solution concentration on the removal of Pb (II) ions by APA and APB (pH = 5.0,amount of adsorbent = 0.1g, contact time = 60 min, shaking speed = 420rpm and temp = 25°C) |
3.9. Effect of Sorbent Dose on Adsorption of Turbidity
- Experiments were conducted with the adsorbent dose of 0.1, 0.2, 0.3, 0.4and 0.5g per 50mL of turbid water of 206.75NTU. When the addition of the adsorbent dose increased, the percentage removal of turbidity also increased as shown in fig 9. Adsorption of solid particles increased due to increase in number of active sites that attracts both charged and uncharged particles as the adsorbent dose increased. 0.1 g of the APA reduces the turbidity of 50mL of 206.75NTU by 98.62% and APB by 98.57%. Increase in dosage increases the percentage removal of turbidity similar to the findings reported by Thirugnanasambandham et al. [14].
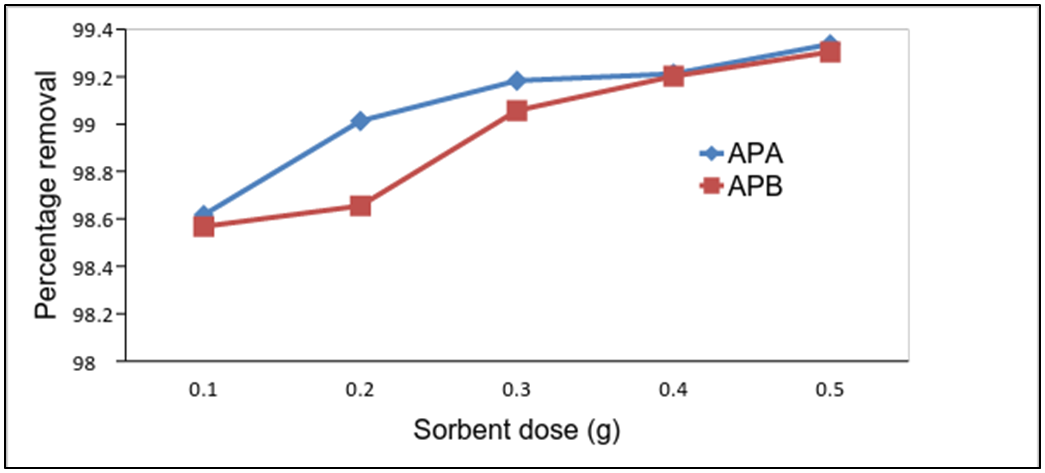 | Figure 9. Effect of sorbent dose on the removal of turbidity by APA and APB (initial turbidity = 206.75NTU and temp = 25°C) |
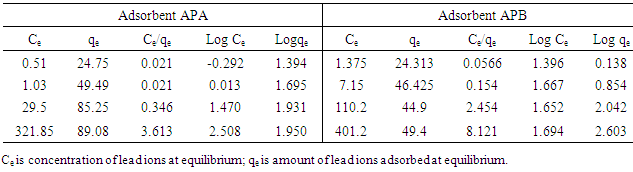
3.10. Langmuir and Freundlich Isotherm Constants for Adsorption of Lead Ions
- As shown in Table 3, adsorption data for lead ions using APA and APB were fitted in both Langmuir and Freundlich models. Figure 10 and 11 shows Langmuir isotherms while figure 12 and 13 shows Freundlich isotherms when using adsorbent APA and APB respectively.
|
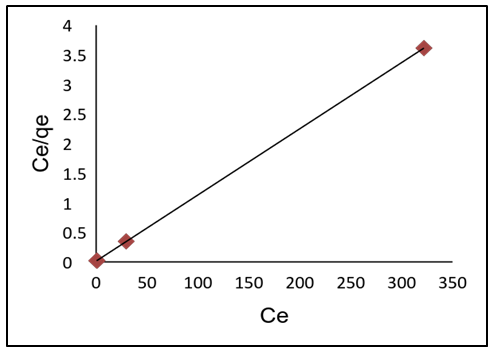 | Figure 10. Langmuir adsorption isotherm plot for Pb (II) adsorption of APA |
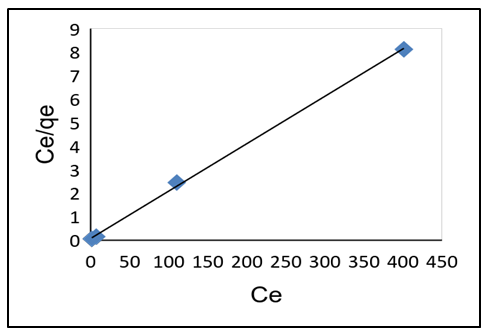 | Figure 11. Langmuir adsorption isotherm plot for Pb (II) adsorption of APB |
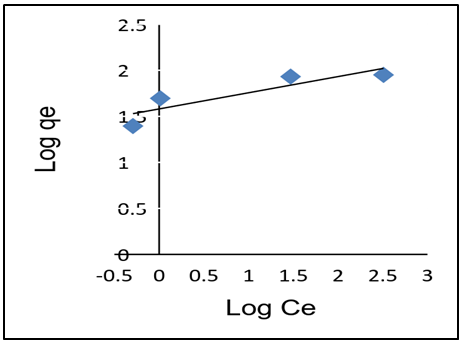 | Figure 12. Freundlich adsorption isotherm plot for Pb (II) adsorption of APA |
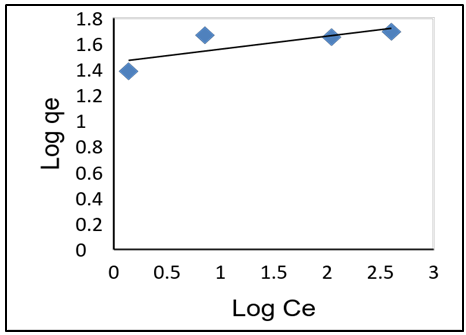 | Figure 13. Freundlich adsorption isotherm plot for Pb (II) adsorption of APB |
|
4. Conclusions
- Adsorbent prepared from TLA and PSA has been explored as a good adsorbent to effectively remove lead ions and solid particles from aqueous medium/ industrial wastewater at ambient temperature. The experimental data showed that under studied conditions the sorption is directly dependent on the mass of adsorbent, temperature, contact time, shaking speed pH and initial metal ions concentration. Therefore, present study shows an economic and prominent alternative towards the wastewater treatment technology as a way of attaining better environmental sustainability. It will also be used to encourage farmers to grow tea and pumpkin as a way of meeting the need for food security in Kenya and also meet the need for raw materials for synthesis of aluminophosphate. Study on use of other plants as source of aluminium and phosphorous need to be undertaken to foster green chemistry in synthesis of aluminophosphate.
ACKNOWLEDGEMENTS
- We are grateful to Kenyatta University and Chemistry Department for supporting our project.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML