-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Frontiers in Science
p-ISSN: 2166-6083 e-ISSN: 2166-6113
2017; 7(3): 42-45
doi:10.5923/j.fs.20170703.02

Characterization of Biodiesel Produced from Waste Cooking Oil Obtained from Food Vendors within Awka Metropolis
Ugochukwu G. C.1, Eneh F. U.1, Adindu C. S.1, Ojiako O. A.2, Aloh C. H.1, Enemoh C. G.1
1Department of Applied Biochemistry, Nnamdi Azikiwe University, Awka, Nigeria
2Department of Biochemistry, Federal University of Technology Owerri (FUTO), Nigeria
Correspondence to: Ugochukwu G. C., Department of Applied Biochemistry, Nnamdi Azikiwe University, Awka, Nigeria.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

The environmental issues associated with the use of fossil-based energy sources have informed the search for more sustainable energy alternatives. This work investigated the potential of producing biodiesel from waste cooking oil (WCO) collected from eateries and fast food restaurants in Awka, Anambra Nigeria. One-step alkaline (KOH) catalytic process was employed in the production of the biodiesel, which was subsequently characterized using standard analytical chemistry techniques and gas chromatography fitted with a flame ionization detector (GC-FID) for the fatty acid methyl ester (FAME) composition. Maximum biodiesel yield of 92.6% w/w WCO was observed at methanol-oil molar ratio of 6:1 and catalyst concentration of 0.7 KOH wt %. The produced biodiesel was rich in monounsaturated and saturated fatty acid (74.80%) implying that it was stable and less prone to oxidative attack. Other physicochemical parameters for the produced biodiesel fall within the range stipulated by the American Society for Testing and Materials (ASTM). The work gives ample evidence that oil from eateries in Awka could be used in producing high quality biodiesel in an easy, one-step transesterification reaction without the need for acid esterification which increases the overall cost of the production process.
Keywords: Biodiesel, Waste cooking oil, Awka, and Gas chromatography
Cite this paper: Ugochukwu G. C., Eneh F. U., Adindu C. S., Ojiako O. A., Aloh C. H., Enemoh C. G., Characterization of Biodiesel Produced from Waste Cooking Oil Obtained from Food Vendors within Awka Metropolis, Frontiers in Science, Vol. 7 No. 3, 2017, pp. 42-45. doi: 10.5923/j.fs.20170703.02.
Article Outline
1. Introduction
- Currently the global energy demand stands at 9, 301 Mtoe [1] and is largely met by hydrocarbons trapped in fossils formed over several millennia. Increase in the global population, massive urbanization and technological advancements equate rise in global energy demand and therefore a potential increase in fossil fuel consumption; if fossil-based fuels are not substituted by renewable energy sources [2]. The use of fuels from renewable resources as opposed to limited fossil oil sources has gained global interest owing to its potential to lower emission of greenhouse gases and other forms of environmental pollutants [3]. Consequently, international investment in the development of renewable energy forms including hydropower, solar energy, wind energy, biofuels and geothermal energy has gained traction within the last two decades [4, 5].Biodiesel is a diesel fuel produced from plant oils and animal fats. They primarily contain long chain alkyl esters and are very stable [6]. They are also biodegradable, nontoxic and can reduce the rate of emission of environmental pollutants [7]. Current sources of large scale biodiesel production include soybean, rapeseed, palm, and jatropha oils. However, large scale production of biodiesel from these plant sources have come under severe scrutiny as it is feared that it will lead to hike in food prices, loss of agricultural lands and biodiversity [8, 9]. This challenge has informed the use of non-food sources such as microalgae and waste cooking oil (WCO) as an alternative feedstock to economize the biodiesel production process [10]. In this study, the potential for biodiesel production from WCO obtained from fast food restaurants and eateries within Awka metropolis was investigated. This research has twofold benefit of helping contribute to the drive for development of renewable bioenergy and curtailing the disposal of waste oil into drainages where they constitute environmental nuisance.
2. Materials and Method
2.1. Collection and Filtration of the Oil
- The waste cooking oil samples were collected from eight different eateries and fast food joints in Awka, Anambra State, Nigeria. Solid debris in the samples was removed using a filter cloth and funnel, and then stored in air tight plastic containers (away from sunlight) until needed for analysis.
2.2. Physicochemical Characterization of Oil Samples
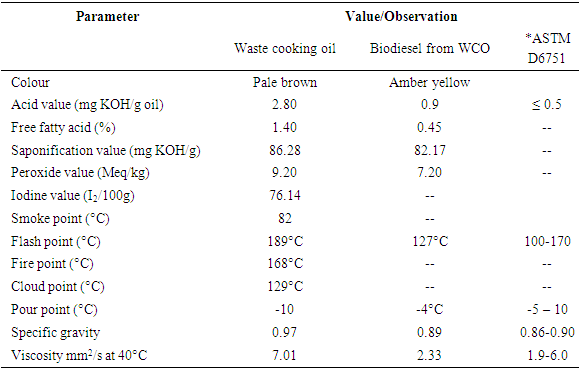
- The WCO samples were mixed together and subsequently characterized. However, before the oils were mixed, their acid values were estimated. Those with acid values above 3 mg KOH/g were not further characterized and hence not used for biodiesel production. The method of Association of Analytical Chemists [11] was employed in the determination of acid value, iodine value, peroxide value, saponification value, flash point, smoke point and cloud point. While the method of American Society for Testing and Materials [12] was used in measuring viscosity and specific gravity.
2.3. Production of Biodiesel
- After physicochemical analysis of the oil samples, those with acid value between 0-3 mg KOH/g oil were used for biodiesel production employing the one-step alkaline transesterification procedure. Alkaline Transesterification Procedure:This was done as described by Antolin et al., [13] and Tiwari et al., [14]. Varying methanol to oil molar ratio of 3:1 to 10: 1 were mixed in a 250 ml round-bottom flask, and 0.55-2.00 wt% of KOH added to the mixture to serve as an alkaline catalyst. The reaction mixture was heated at 50°C for 40 minutes while been gently agitated with a magnetic stirrer. After 40 minutes, the reaction mixture was allowed to stand for 6 hours while phase separation occurred by gravity settling. The biodiesel was carefully decanted into another bottle leaving the glycerol at the base. The biodiesel so produced was cleaned with warm (40°C) distilled water to remove impurities (glycerides, methanol, KOH) as described by Suprihastuti, [15]. Distilled water (50% v/v) was added to the crude biodiesel, stirred for 20 minutes and afterwards left for 2 h for gravity settling. The water was decanted and the residual water left afterwards was removed by heating the biodiesel over anhydrous CaCl2.Yield calculation: the yield was calculated as follows:Biodiesel yield % (w/w) was calculated as =
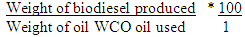
2.4. Fatty Acid Analysis of Produced Biodiesel
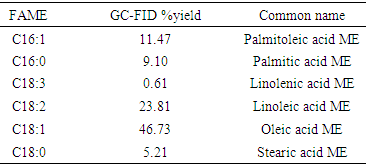
- Fatty acids were analyzed by gas chromatography-flame ionization detector (GC-FID) system equipped with a capillary column (HP-5 MS, 5% phenyl methyl silox 30 m × 250 μm × 0.25 μm nominal) as described by Patil et al., [16]. Methyl heptadecanoate (10.00 mg; internal standard) was dissolved in 1 mL heptane to prepare the standard solution. Approximately 55 mg methyl ester was dissolved in 1 mL standard solution for GC analysis. Approximately 1 μL sample was injected into the GC. Helium was used as the carrier gas. The injection was performed in splitless mode.
3. Results
|
|
4. Discussion
- Biodiesel produced from WCO is increasingly used in powering cars, trucks, and industrial machines that have standard diesel engines, and hence requires no engine conversion. Biodiesel has been demonstrated to have an advantage over its petroleum-derived counterpart because of its lower carbon footprint, and this research evidences the possibility of producing biodiesel from WCO obtained from eateries. The production of biodiesel used in the present research involved a one-step transesterification process as against the two-step process that involves acid esterification using H2SO4 and alkaline transesterification. The latter method is employed when the acid value is high; above 3% wt of the oil [17, 18] requiring that the free fatty acids be neutralized first to enable subsequent transesterification reaction. Of the eight oil samples collected for this research effort, five had acid values below 3 mg KOH/g making them suitable for the experiment; the other two had acid values above the recommended range.The highest yield for the biodiesel in the present research was 92.6% w/w, which was obtained when methanol to oil molar ratio was 6:1, and alkali catalyst concentration of 0.7% KOH. The yield compares favorably with results from previous literatures [19, 20] whose yields were between 80-98% under similar reaction conditions as in the present research. The high yield is attributable to the nature and concentration of the alkaline catalyst used for the reaction process. Generally, alkaline catalyst such as KOH and NaOH give faster esterification time and yield as against acid catalyst. However, they perform poorly if the feedstock is rich in water vapour [21].Most of the physicochemical properties of the produced biodiesel fall within the range stipulated by American Society for Testing and Materials (ASTM) for biodiesels blended with middle distillate fuels. Although the acid value (Table 1; 0.9 mg KOH/g) was higher than the range stipulated by ASTM (≤ 0.5), the free fatty acid content (0.45%) was low enough to enable efficient transesterification. The fatty acid content (Table 2) shows the biodiesel to be rich in the oleic acid (46.73%), a monousaturated fatty acid, with methyl esters of monounsaturated and saturated fatty acids constituting 74.80% of the oil. This implies oil of high stability as monounsaturated/unsaturated fatty acids are less prone to oxidation and rancidity [22].
5. Conclusions
- The quest to discover sustainable energy sources that add little or no net pollution to the environment is one that must be vigorously pursued. In this research, it was demonstrated that waste oils obtained from fast food / restaurants within Awka, Anambra State Nigeria could be used in biodiesel production in a one-step transesterification procedure that is easy to perform. Nonetheless, further work is proposed in the area that will involve spent oil from local food kiosks and peddlers that often reuse oil severally before disposing them, to ascertain the cost effectiveness of the overall process. It is also pertinent that a life cycle analysis (LCA) be carried out to determine the environmental impact of producing biodiesel from waste vegetable oils obtained from food vendors.
ACKNOWLEDGEMENTS
- We wish to thank Mr. David Okeke of Springboard Labs, Awka, Anambra Nigeria, for helping with the interpretation of the GC-FID data. We also thank the management of Thrillers and Nourisha Foods (both in Awka, Anambra state) for providing us with WCO, at no cost, to support this research effort.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML