-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Frontiers in Science
p-ISSN: 2166-6083 e-ISSN: 2166-6113
2017; 7(3): 37-41
doi:10.5923/j.fs.20170703.01

Physical and Environmental Studies on Poly(isobutylene-alt-maleic acid) Derivative/ Polypropylene Multi-Walled Carbon Nanotube-based Filaments
1Nanoscience and Technology Department, National Centre for Physics, Islamabad, Pakistan
2Department of Chemistry, Quaid-i-Azam University, Islamabad, Pakistan
Correspondence to: Ayesha Kausar, Nanoscience and Technology Department, National Centre for Physics, Islamabad, Pakistan.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

In this endeavor, a novel blend of poly[(isobutylene-alt-maleic acid, ammonium salt)-co-(isobutylene-alt-maleic anhydride)]/polypropylene (PIMA/PP) was designed and reacted with functional multi-walled carbon nanotube (MWCNT). Nanotube was functionalized to generate carboxyl group on surface and then reacted with PIMA/PP to make them interact with the blend. Wet-method was used to form PIMA/PP/MWCNT nanocomposite filaments with various nanofiller content. Acid as well as polymer functionalization of MWCNT and interaction between nanofiller and matrix was confirmed using FT-Raman spectroscopy. Functionalization also played an important role to improve nanotube dispersion in the matrix. According to DSC analysis, crystallinity property and melting temperature of PIMA/PP/MWCNT nanocomposite was found to increase with the nanotube incorporation. The nanocomposite also showed fine metal ion sorption with time and nanotube loading.
Keywords: Polypropylene, MWCNT, Functionalization, Sorption
Cite this paper: Ayesha Kausar, Physical and Environmental Studies on Poly(isobutylene-alt-maleic acid) Derivative/ Polypropylene Multi-Walled Carbon Nanotube-based Filaments, Frontiers in Science, Vol. 7 No. 3, 2017, pp. 37-41. doi: 10.5923/j.fs.20170703.01.
Article Outline
1. Introduction
- Polypropylene (PP) is an important thermoplastic with huge range of industrial and commercial uses [1-3]. PP possess several valuable properties such as low cost, fine processability, chemical stability, and ease of fabrication. Similarly, poly(isobutylene-alt-maleic acid) and its derivatives are important thermoplastics [4, 5]. Various type of nanofillers have been used for thermoplastic polymer including carbon nanotube, nanodiamond, graphene, graphite, carbon black, etc. [6-9]. Carbon nanotube is a widely used nanofiller in polymer nanocomposites [10-13]. It was first time discovered by Ijima in 1991. Carbon nanotube possess concentric layered tube-like structure made up of hexagonally arranged carbon atoms. Carbon nanotube can be widely classified as single-walled carbon nanotube (SWCNT) and multi-walled carbon nanotube (MWCNT) [14, 15]. Single-walled carbon nanotube (SWCNT) consist of sheet of atomically thin carbon i.e. rolled in the form of cylinder. In multi-walled carbon nanotube (MWCNT), several graphene layers are rolled in concentric cylinders. CNT have diameters ranging from one nanometer to tens of nanometers and length in micrometer range. Among these nanotube, multi-walled carbon nanotube have been extensively used in polymer nanocomposite due to easy production and low cost compared with SWCNT. Moreover, MWCNT own range of advantageous mechanical, electrical, and other physical properties to be employed as nanofiller in polymers [16, 17]. In thermoplastics, poor dispersion and weak interfacial bonding are the main drawbacks limiting the nanotube dispersion in matrix. Various attempts have been made to overcome the technical difficulties and for realizing the full potential of nanotube as reinforcing agents. One of the most effectual ways of increasing the dispersibility is the chemical functionalization of CNT. Functionalization enhances interfacial bonding between the nanotube and polymer interface. The interfacial binding also improves the load transfers from a polymer matrix to the CNT. Therefore, it is indispensable to functionalize the nanotube in order to exploit their merit in polymer nanocomposites [18, 19]. A significant use of thermoplastics and carbon nanotube is metal ions sorption from waste water. In this regard, various polymer and nanofiller-based nanocomposites and membranes have been reported [20-22]. Owing to the potential of polymer/CNT nanocomposite, poly[(isobutylene-alt-maleic acid, ammonium salt)-co-(isobutylene-alt-maleic anhydride)]/ polypropylene (PIMA/PP) matrix have been developed and reacted with functional MWCNT. Firstly, functionalization on MWCNT has been achieved by introducing carboxylic acid group (-COOH) using nitric and sulfuric acid oxidation. Dispersion of non-functional MWCNT in polymers was difficult. The aim of this study was to disperse MWCNT in PIMA/PP and increase the interfacial bonding between the nanotube and matrix. In addition, a fiber system was prepared with PIMA/PP/MWCNT nanocomposite. The structure, dispersion, and thermal properties of the fiber system were examined at different MWCNT concentration. Metal ion sorption for arsenic uptake of nanocomposite was studied for the removal of water pollution.
2. Experimental
2.1. Materials
- Poly[(isobutylene-alt-maleic acid, ammonium salt)-co-(isobutylene-alt-maleic anhydride)] (PIMA, average Mw ~60,000), polypropylene (PP, average Mw ~12,000), tetrahydrofuran (THF, 99.5%), xylene (97%), nitric acid (HNO3, 70%), sulfuric acid (H2SO4, 99.99%), and multi-walled carbon nanotube (MWCNT, O.D.×L 6-9 nm×5μm, >95%) were bought by Aldrich.
2.2. Instrumentation
- Infrared (IR) spectra were recorded on Fourier transform infrared (FTIR) Spectrometer, FTSW 300 MX (BIO-RAD). Raman spectra were carried out on Raman spectrometer (Bruker). Differential scanning calorimetry (DSC) was performed using Perkin-Elmer DSC (Boston, MA, USA). Few mg of samples were analyzed at heating rate of 10 °C/min under nitrogen flow. Metal ion sorption studies were performed to study the percent metal ion uptake (arsenic ions).
2.3. Purification of Raw MWCNT
- Purification of MWCNT involves annealing of 5g raw MWCNT at 500°C for 0.5 h. The annealed MWCNT was sonicated by adding 500 mL HCL in a 500mL two necked round bottom flask. The mixture was sonicated for 2h at room temperature. The nanotube was filtered through suction filtration assembly, followed by washing with distilled water to attain pH ~7. As filtered MWCNT was dried at 80°C for 8 h [23].
2.4. Carboxylic acid Functionalization of Multi-walled Carbon Nanotube (MWCNT-COOH)
- 5 g MWCNT was added to 100 mL H2SO4:HNO3 mixture (3:1). The mixture was sonicated and refluxed for 6 h at 60°C. The mixture was filtered and washed with distilled water to attain pH~7. The MWCNTs-COOH was dried at 80°C for 12 h. FTIR (KBr) 3426 (O–H stretching vibration), 1720 cm-1 (C=O stretching vibration) [24].
2.5. Formation of Poly[(isobutylene-alt-maleic Acid, ammonium salt)-co-(isobutylene-alt-maleic anhydride)]/ Polypropylene (PIMA/PP) Blend
- PIMA (0.1 g) and PP (0.1 g) was dissolved in 100 mL THF and stirred for 1hr in Xylene (100 mL). The blend mixture was refluxed for 2 h to attain homogeneous mixture [25, 26].
2.6. Functionalization of Multi-walled Carbon Nanotube with Poly[(isobutylene-alt-maleic Acid, Ammonium salt)-co-(isobutylene-alt-maleic anhydride)]/polypropylene Blend (PIMA/PP/MWCNT)
- The acid treated MWCNT (0.05 g) were ultrasonicated in THF (100 mL) for 6h. PIMA/PP dissolved and stirred in 100 mL THF under reflux. The MWCNT suspension was dropped into the PIMA/PP solution and stirred for 1h. During this process, MWNT-COOH interacted with the PIMA/PP blend (Fig. 1). Finally, the solvent of the mixture was removed using a rotary evaporator. A dark grayish powder was obtained as product.
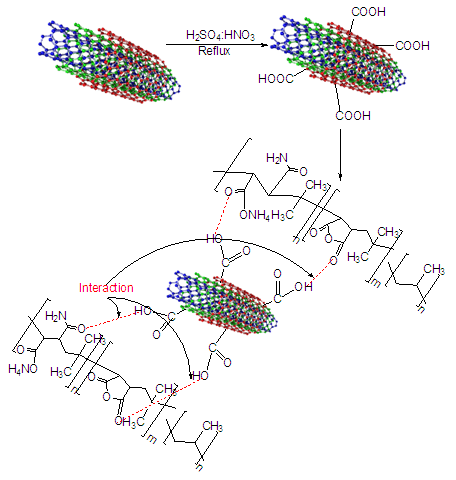 | Figure 1. Schematic diagram of interaction between PIMA/PP/MWCNT |
2.7. Preparation of PIMA/PP/MWCNT Nanocomposite Filament
- PIMA/PP/MWCNT nanocomposite was prepared by wet blending of PIMA/PP with given ratio of MWCNT (0.1, 0.5, 1.0 wt. %). The nanocomposite solution was passed through a multi-hole spinneret. The solution coming out from the spinneret was poured in ethanol: water (1:1) mixture. The PIMA/PP/MWCNT nanocomposite filaments were extracted and dried at 80°C for 6h and further annealed at 120°C for 2 h [27-30].
3. Result and Discussion
3.1. Dispersion of Pre-treated MWCNT
- By means of acid treatment, neat MWCNT was functionalized with carboxylic groups, which may contribute to the enhanced nanotube dispersion in water or organic medium. The MWCNT were ultrasonicated in ethanol and THF. Fig. 2 shows solution containing neat nanotube, MWCNT-COOH, and PIMA/PP/MWCNT dispersion. The neat MWCNT ultrasonicated in ethanol settled immediately, as shown in Fig. 2A. The acid functionalized MWCNT remained dispersed in the ethanol (Fig. 2B). The PIMA/PP/MWCNT were well-dispersed in ethanol and remained stable for more than 48 h (Fig. 2C). Similar results were obtained for THF, which was used as the solvent to react MWCNT-COOH with polymer.
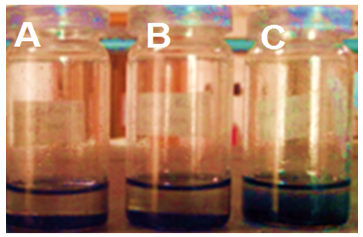 | Figure 2. Dispersion of (A) neat MWCNT; (B) acid treated MWCNT; and (C) PIMA/PP/MWCNT obtained at 510 nm |
3.2. FT-Raman
- Fig. 3A shows two major peaks in the high frequency range. The tangential mode i.e. G-band appeared at 1579 cm-1 and D-band at 1350 cm-1. These bands were assigned to carbonaceous compounds or defects in nanotube surface. The ratio of the intensity of D-band to G-band in MWCNT-COOH was proof for sidewall functionalization [31]. Increase in sp3-hybridized carbon atoms advocates that some species are bonded covalently to nanotube framework. In acid treatment, the ratio was increased from 1.0 for the neat MWCNT to 1.2 for acid treated MWCNT (Fig. 3B). The PIMA/PP/MWCNT nanocomposite showed strong absorbance for polymer at 1780 cm-1. The carboxylic acid C=O stretch was observed at 1715 cm-1 (Fig. 3C). This was attributed to the hydrogen bonding between anhydride and MWCNT-COOH, as given in Fig. 1 [32].
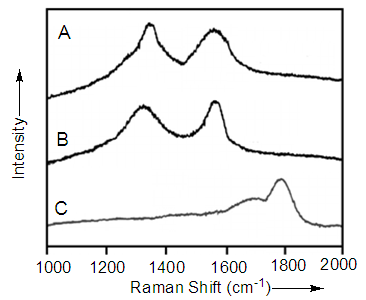 | Figure 3. FT-Raman spectra of (A) neat MWCNT; (B) acid treated MWCNT; and (C) PIMA/PP/MWCNT obtained at 514.5 nm |
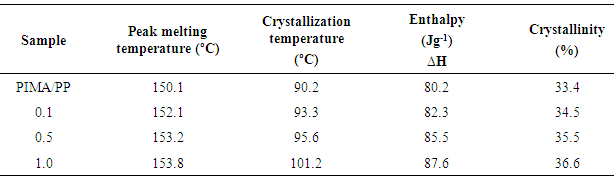
3.3. DSC
- DSC measurements were performed to determine the thermal properties of PIMA/PP/MWCNT nanocomposite. The results show that the heat of fusion of matrix with MWCNT was slightly higher than that of the sample without nanotube content. The results suggested that more ordered polymer packing was obtained for PIMA/PP/MWCNT nanocomposite. Moreover, the presence of MWCNT enhanced the crystallization process. The change in crystallinity was because of nanotube incorporation (Table 1). The crystallization in polymer involves primary nucleation and rapid spherulitic growth [33-35]. The crystallinity was calculated using equation (1).
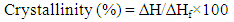 | (1) |
|
3.4. Metal Ion Sorption
- The PIMA/PP/MWCNT filament mat was used for toxic ion removal. The percent metal ion (As5+) uptake as function of time with filler content is shown in Fig. 4. The nanofiber mat specimen (0.1 g) was placed in flask containing 10.0 mL solution of arsenic ions. Initial concentration of ions was 100 ppm. Each flask was shaken in thermostatic shaker bath at 30°C and 100 rpm. 1 mL of test solution was withdrawn at 24 h and diluted with distilled water. The sample solutions was quantified for the amounts of metal ions using Varian SpectrAA-300 atomic absorption spectroscope (AAS). The metal ion sorption capacity of PIMA/PP/MWCNT nanocomposites was enhanced with the increase in nanotube content sorption time. Maximum absorption was perceived after 120 min. The enhanced sorption was because of restricted diffusion of the ions through polymer/nanotube filament network and structural rigidity.
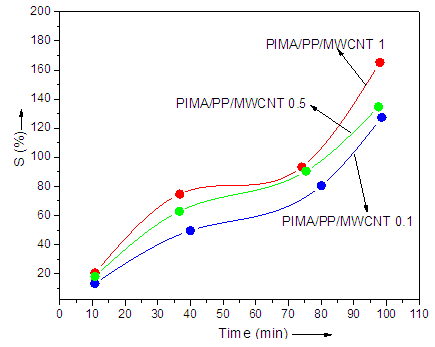 | Figure 4. Effect of nanofiller content and sorption time on percent arsenic uptake |
4. Conclusions
- In this work, a novel method was defined to make PIMA/PP/MWCNT nanocomposite filaments with various nanofiller content. Multi-walled carbon nanotube was first functionalized to have carboxyl group. Afterthat, MWCNT was reacted with PIMA/PP in THF solvent to make them interact with a blend of polypropylene. The functionalized MWCNT was mixed with matrix by wet spinning method and resulting filaments were drawn, and annealed. FT-Raman spectra showed that the functionalized MWCNT were well dispersed in matrix due to the hydrogen bonding between anhydride and MWCNT-COOH. The interfacial interaction frolicked important role in improving the nanotube dispersion. In addition, crystallinity property was affected by the incorporation of MWCNT in matrix. Metal ion sorption study showed that the nanotube formed fine network with the matrix in filament structure. Novel PIMA/PP/MWCNT nanocomposite may perform important role for environmental remediation of municipal and industrial wastewater. These results also suggest that wet-spinning method was effective in preparing high performance nanocomposite.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML