-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Frontiers in Science
p-ISSN: 2166-6083 e-ISSN: 2166-6113
2017; 7(2): 31-35
doi:10.5923/j.fs.20170702.02

Polymer/Silver Nanoparticle Nanocomposite as Antimicrobial Materials
1Nanoscience and Technology Department, National Centre for Physics, Islamabad, Pakistan
2Department of Chemistry, Quaid-i-Azam University, Islamabad, Pakistan
Correspondence to: Ayesha Kausar, Nanoscience and Technology Department, National Centre for Physics, Islamabad, Pakistan.
| Email: |  |
Copyright © 2017 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

This article shows an attempt to prepare poly(acrylonitrile-co-methyl acrylate)/polyaniline (PANMA/PANI)-based nanocomposite with silver nanoparticle (AgNP) and cellulose-silver nanoparticle (Cell-AgNP). Silver nanoparticles have been prepared by photoreduction of silver nitrate in UV light in the presence of poly(N-vinylpyrrolidone). Cell-AgNP were then prepared using reflux method. The nanoparticles were characterized by transmission electron microscopy (TEM) and UV-vis spectra. Antimicrobial activity of PANMA/PANI/Cell-AgNP nanocomposites with various nanoparticle content have been tested using exposure time against S.aureus. Both the exposure time and nanoparticle content in novel nanocomposites affected the antimicrobial activity.
Keywords: Polyaniline, Silver nanoparticle, Cellulose, Antimicrobial
Cite this paper: Ayesha Kausar, Polymer/Silver Nanoparticle Nanocomposite as Antimicrobial Materials, Frontiers in Science, Vol. 7 No. 2, 2017, pp. 31-35. doi: 10.5923/j.fs.20170702.02.
Article Outline
1. Introduction
- Nanoparticle synthesis for various applications is an interesting field [1-3]. Both organic as well as inorganic nanoparticles have been focused in this regard [4-8]. These nanostructures parade novel properties which largely differ from the bulk properties. Reactivity of these small entities strongly be contingent on particle size, shape, and surface area [9-17]. Various type of metal nanoparticles have been prepared using advance techniques. Nanoparticle modification with other nanoparticles is also an important research filed [18-24]. One of the method is reduction of metal ions to form protective colloids. Water-soluble polymers such as poly(vinyl alcohol), poly(N-vinylpyrrolidone), poly(methyl vinyl ether), etc. have been used for the preparation of metal particles. Among metal nanoparticles, silver nanocrystallites are predominantly fascinating due to exciting characteristics. Various reductants have been used to prepare colloidal silver in the presence of copolymers. Another successful method for the preparation of Ag nanoparticles is in the presence of UV irradiation and poly(N-vinylpyrrolidone). An important property of silver ions is bacteriostatic/ bactericidal nature. Therefore, silver nanoparticle (AgNP) have been used as antibacterial agent to increase bacterial resistance to conventional bactericides and antibiotics. The antimicrobial activity of silver nanoparticles combined with chitosan or cellulose have been rarely studied [25-33]. Moreover, cellulose/silver nanoparticle in polymer nanocomposite can be employed as biocompatible materials with enhanced antimicrobial activity. Polymer nanocomposites with nanoparticles also gained wide scope for technological significance. In this work, stable silver nanoparticle has been prepared by reduction of silver nitrate with UV light and poly(N-vinylpyrrolidone). The AgNP have been characterized by transmission electron microscopy (TEM) and UV-vis spectra. Moreover, their antimicrobial activity has been tested in the form of poly(acrylonitrile-co-methyl acrylate) nanocomposites. The main objective of this study was to perceive the effect of silver nanoparticle and cellulose/silver nanoparticle in the form of poly(acrylonitrile-co-methyl acrylate) nanocomposites.
2. Experimental
2.1. Materials
- Poly(acrylonitrile-co-methyl acrylate) (acrylonitrile ~94 wt. %), poly(vinylpyrrolidinone) (PVP), polyaniline (emeraldine base) average (Mw ~100,000), cellulose, and dimethylacetamide (DMAc, 99%) were purchased from Aldrich.
2.2. Measurements
- UV-2600/2700 UV-VIS spectrophotometer by Shimadzu was used to analyze the samples. Transmission electron microscopy (TEM) was conducted with a JEOL JEM 2100F TEM. The sample was prepared with a Leica UC-6 ultramicrotome with a Diatome diamond knife at room temperature. Antibacterial activity was studied using spread plate method.
2.3. Formation of Silver Nanoparticle (AgNP)
- 3 mL of aqueous AgNO3 (0.2 mM) containing 0.2 wt. % PVP was placed in a quartz vessel. The sample was irradiated by 254 nm UV light with four 40 W low-pressure mercury lamps at room temperature [34].
2.4. Cellulose-Silver Nanoparticle (Cell-AgNP)
- 0.5 g cellulose was stirred in 50 mL DMAc for 3h. 0.5 g AgNP (obtained in previous Section) was dispersed in DMAc (50 mL) separately using sonication of 3h. Then both the dispersions were mixed and refluxed for 12 h at 110°C. The stirred suspension was allowed to settle and the supernatant was discarded. The product was dried at 80°C for 24 h [35].
2.5. Preparation of Poly(Acrylonitrile-Co-Methyl Acrylate)/Polyaniline/Cellulose-Silver Nanoparticle (PANMA/PANI/Cell-AgNP) Nanocomposite
- The nanocomposites were prepared by reflux method using DMAc at 130°C for 6 h. Firstly, a 90:10 blend of poly(acrylonitrile-co-methyl acrylate)/polyaniline was prepared by refluxing in DMAc at 120°C. The poly(acrylonitrile-co-methyl acrylate)/polyaniline were mixed with various weight percent of Cell-AgNP (Table 1). The nanocomposite was collected by precipitation method in xylene. The product was dried at 80°C for 24 h [36].
|
3. Results and Discussions
3.1. Silver Nanoparticle and Cellulose-Silver Nanoparticle
- UV-vis absorption spectra are proved to be sensitive to the formation of silver colloids. Fig. 1 compares the absorption spectra obtained for AgNP prepared from 0.2 mM AgNO3 and 0.2 wt. % PVP after complete photoreduction using 254 nm UV light and cellulose-silver nanoparticles. An intense absorption peak around 401 nm was observed due to surface plasma excitation of silver particles (Fig. 1A). The peak position for Fig. 1B was determined to be at 410 nm corresponding to cellulose addition. Therefore, the peak was shifted toward red in pure AgNP as demonstrated in the figure. The colloidal silver, thus, caused a decrease in the intensity of the main peak around 400 nm. There was one symmetric absorption peak around 400 nm due to primary dipolar excitation [37, 38].
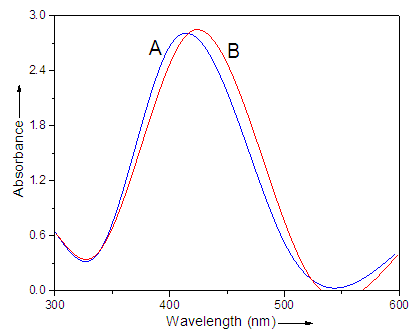 | Figure 1. UV-vis spectra of (A) silver colloids and (B) Cell-AgNP prepared from UV reduction of 0.2 mM AgNO3 in PVP |
3.2. Transmission Electron Microscopy
- TEM analysis was carried out as a characterization method to evaluate the morphology of nanoparticles and also their behavior in the matrix. Silver nanoparticles depicted uniform morphology with smooth surface and homogeneous distribution (Fig. 2A). Addition of cellulose to the AgNP caused increase in the particle size (Fig. 2B). The distribution of the nanoparticle was also not that uniform in the case of Cell-AgNP. TEM images of the 3 wt. % PANMA/PANI/Cell-AgNP nanocomposite prepared are presented in Fig. 2C. As can be seen from micrograph, few aggregation particles were visible in the blended nanocomposite. The system appears to be a phase separated morphology. However, the overall morphology of nanocomposites showed good dispersion of the nanoparticles. This is a typical blend morphology for nanoparticles in a polymer blend matrix [39].
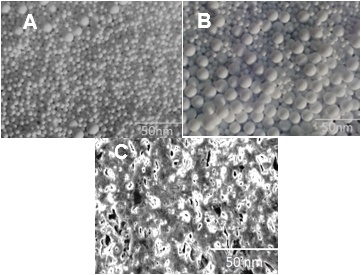 | Figure 2. TEM micrographs of (A) silver colloids; (B) Cell-AgNP; and (C) PANMA/PANI/Cell-AgNP 3 nanocomposite |
3.3. Antimicrobial Activity
- Evaluation of antibacterial activity of PANMA/PANI/Cell-AgNP 1-3 nanocomposite was performed using the conventional spread plate method. Fig. 3 shows plots of log (sample remained) versus exposure time against S.aureus. The sample tend to interact strongly with the media with cultivated bacteria in agar plate. The polymeric chains interact with charged species of the bacterial media. It was observed that the PANMA/PANI/Cell-AgNP 3 nanocomposite with higher nanoparticle content was remained or survived the most after the antibacterial test. Therefore the antibacterial activity of Cell-AgNP nanoparticle was verified [40-44].
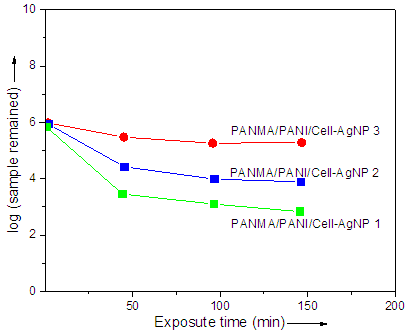 | Figure 3. Plots of log (sample remained) versus exposure time for S. aureus |
4. Conclusions
- In this paper, a blend of poly(acrylonitrile-co-methyl acrylate) and polyaniline has been reported. Afterwards, the nanocomposite with silver nanoparticle and cellulose-silver nanoparticle were prepared and analyzed. Silver colloids were prepared by photoreduction of silver nitrate with UV light in the presence of poly(N-vinylpyrrolidone). Both particle size and the UVvis absorption peak were strongly dependent on PVP and cellulose addition. The antimicrobial activity of nanocomposite with combination of silver and cellulose was found to be satisfactory for technical relevance.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML