-
Paper Information
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Frontiers in Science
p-ISSN: 2166-6083 e-ISSN: 2166-6113
2016; 6(1): 25-30
doi:10.5923/j.fs.20160601.03

Reduction of Anti-nutritional Factors of Sorghum by Lactic Acid Bacteria Isolated from Abacha- an African Fermented Staple
S. M. Adeyemo1, A. A. Onilude2, D. O. Olugbogi1
1Food and Industrial Microbiology Unit, Department of Microbiology, Obafemi Awolowo University, Ile-Ife, Nigeria
2University of Ibadan, Nigeria
Correspondence to: S. M. Adeyemo, Food and Industrial Microbiology Unit, Department of Microbiology, Obafemi Awolowo University, Ile-Ife, Nigeria.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

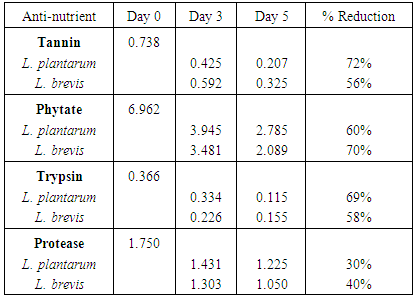
The provision of a balanced weaning food is a major challenge in Africa, especially among low earners. Most women result to fermented cereals fermented by Lactic Acid Bacteria. Cereals contain different anti-nutrients which prevent growth and healing; block the absorption of nutrients and acts as toxins in food. This study focuses on the use of α-galatosidase produced by lactic acid bacteria isolated from fermented Abacha to reduce the anti-nutrients present in sorghum and improve nutrient composition. Replicate samples of Abacha were collected at different time intervals and eighteen strains of lactic acid bacteria were isolated from them. The isolates include Lactobacillus casei, L. plantarum, L. cellobiosus, L. fermentum, L. acidophilus and L. brevis. Anti-nutritional factors and alpha-galactosidase were determined by UV-spectrophotometry. Two of the strains with the highest production of α-galactosidase, L. plantarum and L. brevis were used separately as starter culture to ferment sorghum. The effects of fermentation at 0, 72 and 120 hours on trypsin inhibitor, protease inhibitor, phytate and tannin, of sorghum were assessed. 69% reduction of trypsin inhibitor; 30% of protease inhibitor; 60% of phytate and 72% of tannin was observed at 120h with L. plantarum used as starter culture while 58% reduction of trypsin inhibitor; 40% of protease inhibitor; 70% of phytate and 56% of tannin was recorded at 120h using L. brevis as starter. Fermentation using LAB as starter culture resulted in reduction in anti-nutritional content of sorghum thereby enhancing it nutritional composition. This probiotic effect can be employed in the production of a good weaning food for infants.
Keywords: Anti-nutrients, Lactic acid bacteria, Microbial fermentation, Sorghum, Enzyme production
Cite this paper: S. M. Adeyemo, A. A. Onilude, D. O. Olugbogi, Reduction of Anti-nutritional Factors of Sorghum by Lactic Acid Bacteria Isolated from Abacha- an African Fermented Staple, Frontiers in Science, Vol. 6 No. 1, 2016, pp. 25-30. doi: 10.5923/j.fs.20160601.03.
Article Outline
1. Introduction
- Lactic Acid Bacteria (LAB) are Gram positive, fastidious, acid tolerant, generally non-sporulating, catalase negative, devoid of cytochrome, and non-respiring rod or cocci that are associated by their common metabolic and physiological characteristics that produce lactic acid as a major or sole product of fermentative metabolism [1]. LAB are the most important bacteria used in food fermentations. Some potential benefits may result from growth and action of the bacteria during the manufacture of cultured foods or in the intestinal tract following ingestion of foods containing them. [2]. Lactobacillus plantarum has been used for the preservation of food for increased shelf life and flavor to get the desired aroma in food [3]. L. plantarum is one of the lactic acid producing bacteria that have been used for centuries for the preservation of human food. It is a simple, safe method that is still used in many undeveloped countries. In addition, scientists conducting different research stated that lactic acid fermentation, such as that used with L. plantarum, is the safest way to preserve food. It is one of the most versatile probiotics. L. plantarum has also been implicated in the lowering of anti-nutrients and unwanted materials in food during fermentation [4], [5]. Sorghum is considered as a most important food crop in the world, following wheat, rice, maize and barley. Unfortunately, sorghum has low nutritional value and inferior organoleptic qualities due to the presence of anti-nutritional factors which make complexes with food ingredients [6].[7] revealed that anti-nutritional factors also known as anti-nutrients are poisonous substances that can be found in most food and able to limit the nutrient available to the body.Many traditional methods of food preparation such as fermentation, cooking, and malting increase the nutritive quality of plant foods through reducing certain anti-nutrients such as phytic acid, polyphenols, and oxalic acid [8]. Traditional cereal fermentation is a spontaneous process initiated by micro flora of the raw materials. It reduces anti-nutritional factors, increases protein digestibility, positivelyenhance texture, aroma and improve the biological value of fermented foods [9]. Tannin, protease and trypsin inhibitor content has also been reportedly reduced by fermentation [10].
2. Materials and Methods
2.1. Sample Collection
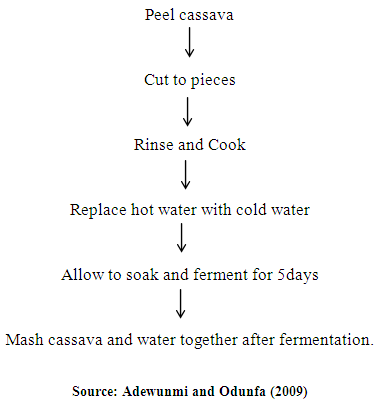
- Cassava used for the preparation of Abacha was harvested from Ondo town and the sorghum was purchased from a local market in Ondo town. Abacha was prepared as follows:
2.2. Isolation and Identification of Lactic Acid Bacteria
- Lactic acid bacteria (LAB) were isolated from Abacha - an Africa staple prepared in Ile- Ife, Osun State. De Man Rogosa Sharpe (MRS) agar was used for the isolation of Lactic acid bacteria. One ml each was taken from the five replicate samples of Abacha and diluted serially at different time intervals. The pour plate technique was used for isolation according to [1]. The plates were incubated anaerobically at 37°C for 48 h and the isolates were purified by successive streaking on MRS agar. Pure LAB isolates were identified and characterized based on morphological and standard biochemical tests which include Gram stain, catalase test, oxidase test, acid production from glucose, motility test and growth at 20°C and 70°C among others.
2.3. Determination of Alpha-Galactosidase
- Alpha-galactosidase activity of the different strains of the LAB isolated were determined. The assay medium consisted of 200µl of 100mM sodium acetate buffer pH 5.0, 2.5ml of 2mM PNP-alpha Gal solution and 0.5ml of enzyme preparation. The assay was carried out for 15min at 50°C and stopped by the addition of 1ml of 0.5M sodium carbonate. The amount of para-nitrophenyl-α-D- galactophyranoside (PNP-α-G) released was determined at 410nm by taking the absorbance. Undiluted mixture treated in the same way was used to set the spectrophotometer to zero.*One unit of enzyme activity was defined as the number of micro molecules of p-nitrophenyl liberated from PNP-G per ml of cells as read from a standard curve.
2.4. Preparation of Starter Culture for Fermentation
- Starter culture was prepared by inoculating each of the selected organisms in 9ml of sterile MRS broth and incubated at 37°C for 24 h. Two batches of five grams each of surface sterilized sorghum samples were weighed in triplicates into screw capped bottles containing 10ml each of sterile distilled water. 1ml each of the standardized inocula of L. plantarum and L. brevis was added separately to the two batches of sorghum and allowed to ferment for 5 days. Samples were taken for anti-nutritional content determination every 24h. A control was set up for the samples with additional 1ml of sterile distilled water in the samples without the organisms.
2.5. Determination of Anti-Nutritional Factors
2.5.1. Determination of Tannin
- One gram of each sample was weighed into a beaker. Each was soaked with a solvent mixture (80mls of acetone and 20mls of glacial acetic acid) for 5 hours to extract tannin. The samples were filtered through a double layer filter paper to obtain the filtrates which were stored for further use. A standard solution of tannic acid was prepared ranging from 10ppm to 30ppm. The absorbances of the standard solution as well as that of the filtrates were read at 500nm on a spectrophotometer [11].
2.5.2. Determination of Phytate
- Two gram of each sample was weighed into 250mls conical flask. 100mls of 2% concentrated hydrochloric acid was used to soak each sample in a conical flask for 3 hours. This was filtered through a double layer of hardened filter paper. 50mls of each filtrate was placed in 250mls beaker and 107mls of distilled water was added in each case to give proper acidity. 10mls of 0.3% Ammonium Thiocyanate solution was added into each solution as indicator. This was titrated with standard Iron (III) chloride solution, which contained 0.00195g iron per ml. The end point is slightly brownish yellow, which persisted for 5 minutes. The percentage phytic acid was calculated using the formula:
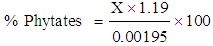 Where X = Titre value (AOAC, 2000)
Where X = Titre value (AOAC, 2000)2.5.3. Determination of Protease Inhibitor
- Egg albumin 2% solution and 0.1% solution of Bromelain, both in pH 7 phosphate buffer, were prepared. 5 ml of the egg albumin substrate and 1 ml of the Bromelain enzyme was incubated at 55°C for 10 min. 5 ml of 10% TCA was added to stop the reaction. The precipitate was filtered off with Whatman No. 1 filter paper and the absorbance of the filtrate was measured at 280 nm on Spectrophotometer. The entire procedure was repeated but incubating with the enzyme and substrate mixture, i.e. 1 ml of the extract of the material for protease inhibitor determination labeled (As). The absorbance of the filtrate was measured at 280 nm. This was denoted Ai.
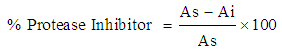 Where As = Absorbance of sampleAi = Absorbance of blank/initial
Where As = Absorbance of sampleAi = Absorbance of blank/initial 2.5.4. Determination of Trypsin Inhibitors
- Two batches each of the samples (0.2 g each) was weighed into a screw capped centrifuge tube. 10 ml of 0.1 M phosphate buffer was added and shaken vigorously. The contents were left at 25°C for 1 h on a UDY shaker. The suspension obtained was centrifuged at 5000 rpm for 5 min and filtered through Whatman No. 42 filter paper. The volume of each was adjusted to 2 ml with phosphate buffer. The test tubes were placed in a water bath, maintained at 37°C. 6 ml of 5% Trichloroacetic Acid (TCA) solution was added to one of the tubes to serve as a blank. 2 ml of casein solution was added to all the tubes, which was previously kept at 37°C. These were incubated for 20 min. The reaction was stopped after 20 min by adding 6 ml of TCA solution to the experimental tubes and shaken. The reaction was left for 1 h at room temperature after which it was filtered through Whatman No. 42 filter paper. Absorbance of filtrate from sample and trypsin standard solutions was read at 380 nm on a spectrophotometer. The trypsin inhibitor in mg/g sample was calculated using the formula
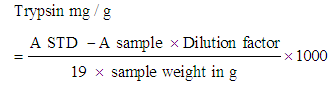 (AOAC, 2000)
(AOAC, 2000)3. Results and Discussion
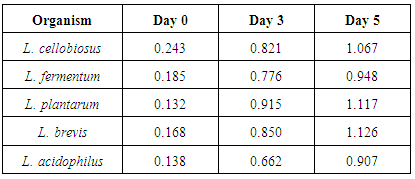
- A total of 18 lactic acid bacteria isolates were isolated from the Abacha samples and were identified as Lactobacillus brevis, L. plantarum, L. fermentum, L. cellobiosus, L. caseiand L. acidophilus. The frequencies of dominance of the Lactobacillus strains in abacha were L. brevis (33.3%), L. cellobiosus (33.3%), L. plantarum (11.1%), L. fermentum (11.1%), L. casei (5.6%) and L. acidophilus (5.6%). This result revealed that traditional fermentation favours the growth of different species of Lactobacillus. This agrees with [12] and [13] on the predominant lactic acid bacteria involved in the traditional fermentation of fufu and ogi. The production of alpha-galactosidase enzyme by the different species of lactic acid bacteria in which Lactobacillus brevis produced the enzyme in abundance (1.126 unit/ml) followed by Lactobacillus plantarum (1.117 unit/ml). This is shown on Table 1 and it agrees with the earlier report of [14].
|
|
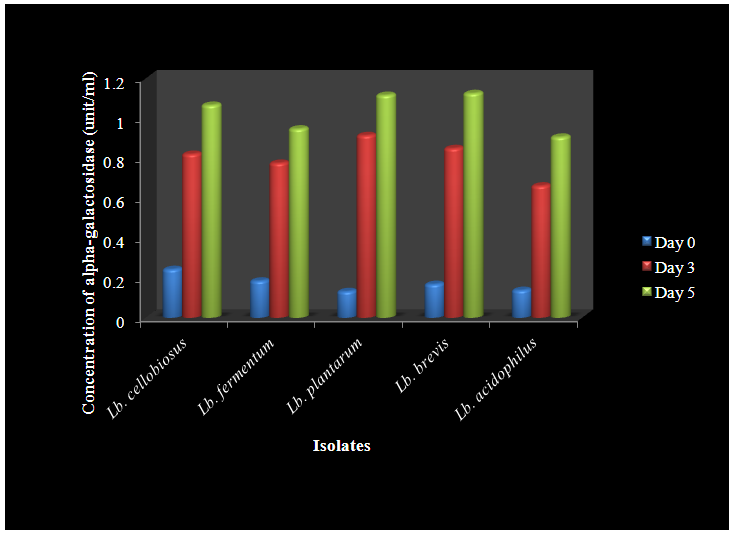 | Figure 1. Production of alpha-galactosidase by LAB isolates |
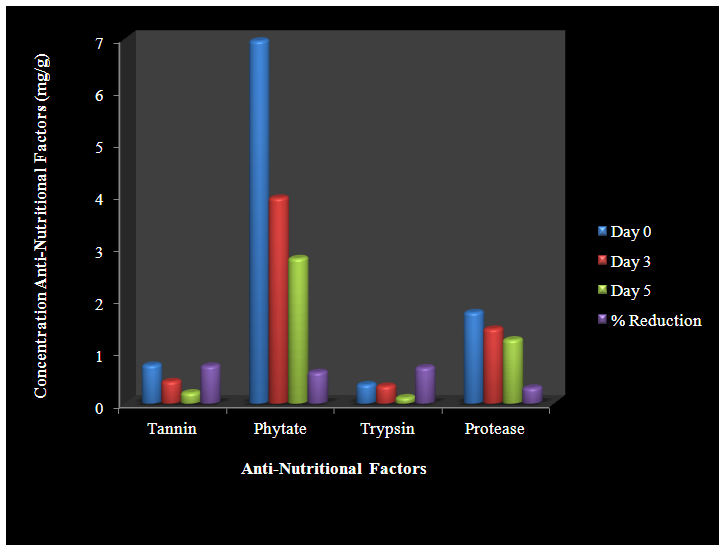 | Figure 2. Reduction of Anti-Nutritional Factors by L. plantarum |
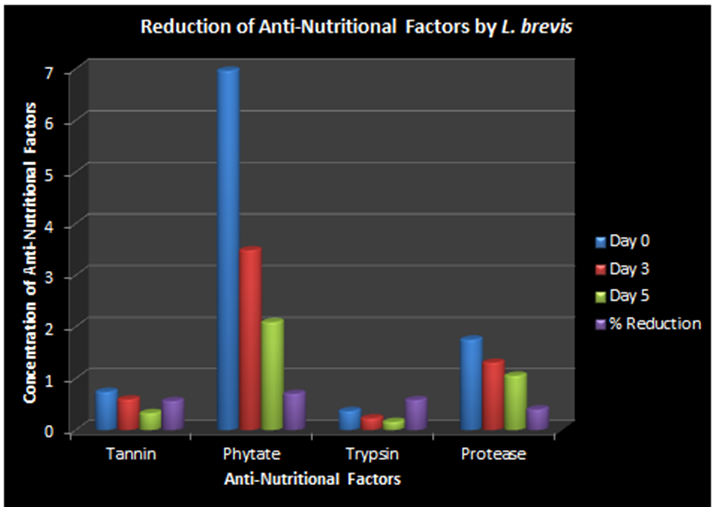 | Figure 3. Reduction of Anti nutritional Factors by Lactobacillus brevis |
4. Conclusions
- This study shows that fermentation using lactic acid bacteria as starter culture resulted in reduction in the anti-nutritional content of sorghum thereby enhancing it nutritional composition. The study showed that sorghum contain some amount anti-nutrients and should be allowed to ferment properly for at least 3 – 5 days to allow for proper growth of lactic acid bacteria that will help in reducing the anti-nutritional factors present in it. The growth of LAB will also inhibit potential pathogens in the fermenting sorghum samples. This is a desirable characteristics of LAB and this probiotic effect can be employed in the production of a good weaning food for infants.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML