-
Paper Information
- Next Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Frontiers in Science
p-ISSN: 2166-6083 e-ISSN: 2166-6113
2016; 6(1): 17-24
doi:10.5923/j.fs.20160601.02

Biosynthesis of Antimicrobial Compounds by Lactic Acid Bacteria and Its Use as Biopreservative in Pineapple Juice
Awojobi K. O., Adeyemo S. M., Sanusi O. O.
Department of Microbiology, Obafemi Awolowo University, Ile-Ife, Nigeria
Correspondence to: Adeyemo S. M., Department of Microbiology, Obafemi Awolowo University, Ile-Ife, Nigeria.
| Email: |  |
Copyright © 2016 Scientific & Academic Publishing. All Rights Reserved.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/

Lactic Acid Bacteria (LAB) are able to synthesize antimicrobial compounds which have both functional and technological properties in foods. This ability of LAB can be explored for use as bio-preservative in food. This study aims at identifying LAB isolated from fresh milk and employing it as natural preservative in extending the shelf life of Pineapple juice. Twenty strains of LAB were isolated from fresh milk and were identified as L. fermentum, L. plantarumand L. lactis respectively. L. fermentum produced the highest antimicrobial compound Lactic acid, diacetyl and hydrogen peroxide (1.8, 0.15, 0.03 g/l) respectively and the least was produced by L. lactis (1.2, 0.10, 0.02 g/l) respectively. Samples were pasteurized at 85°C for 15mins after which lactic cultures were introduced to the fresh pineapple juice singly and in combination. The shelf life and microbial load of the inoculated samples and control without the lactic cultures were monitored at room temperature for 7 days. LAB count in the inoculated samples increased from 3.1x103 - 8.2x106 cfu/ml from day 0 to 5 with no LAB growth noticed in the control. Yeast and Coliform Count increased after day 5 to (5.1x104 and 2.3x103 cfu/ml respectively) but for control increased to 6.3x105 and 5.2x104 respectively. Aerobic plate count increased to 3.0x104 cfu/ml after day 5 and 7.4x105 for control. A decrease was observed in LAB count after day 5 to 3.2x105cfu/ml. Lactic cultures extended the shelf life of Pineapple juice for 5 days after which there was deterioration monitored by increased count and decreased LAB activity. LAB exhibited a high antimicrobial effect on food borne contaminants. This ability of LAB can be employed as biopreservatives against food pathogens which also help to maintain and preserve the nutritive qualities of pineapple juice for an extended shelf life.
Keywords: Lactic acid Bacteria, Shelf life, Antimicrobial Agent, Probiotics, Biopreservation, Microbial load
Cite this paper: Awojobi K. O., Adeyemo S. M., Sanusi O. O., Biosynthesis of Antimicrobial Compounds by Lactic Acid Bacteria and Its Use as Biopreservative in Pineapple Juice, Frontiers in Science, Vol. 6 No. 1, 2016, pp. 17-24. doi: 10.5923/j.fs.20160601.02.
1. Introduction
- Fresh and processed fruits consumption has continued to grow rapidly in recent times, mainly because of the need for a balanced diet, the health benefits, low calories in fruits and the superior flavor of the fresh fruits as compared to canned fruits (Mohammed, 2007, Ragaert et al., 2004). One of the many popular fruit juice products is the pineapple (Ananas comosus) juice. Today, pineapples are marketed as fresh and canned fruits in the United States and other nations and they are mostly used as tropical foods for many recipes including fruit salads, jams, juices and other products (Cho et al., 2004). Pineapples have exceptional juiciness and a vibrant tropical flavor that balances the tastes of sweet and tart. The worldwide total pineapple production is between 16 to 19 million tons (Fernadens et al., 2008; FAO, 2009). The juice from pineapple is widely consumed by both adult and children. Pineapple juice has a proximate composition of 81.2-86.2% moisture, 13-19% total soluble solid of which sucrose, glucose, and fructose are the main components, 0.4% fibre and rich source of vitamin C (Dull, 2000). Lipids and nitrogenous compounds constitute about 0.2%. The shelf life of the juice may be very short if it is not properly preserved. The traditional method of preparation exposes the juice to microbial contamination through various means (Olubukola et al., 2011). One of the possible causes of the short shelf life would be the presence of a high microbial population along the processing chain. Pineapple fruits can be contaminated in the field during harvesting, postharvest handling, and processing, shipping, marketing or in the home (Fernadens et al., 2008). Many microorganisms in particular acid loving bacteria such as some groups of LAB or acid tolerant bacteria such as Staphylococcus sp, Bacillus sp, Pseudomonas sp, Micrococcus sp, Flavobacterium sp etc; fungi such as Aspergillus sp, Mucor sp, Fusarium sp, Penicillium sp; and yeasts such as Geotrichum sp, Candida sp, Saccharomyces sp, Schizosaccharomyces sp; Hanseniaspora sp; Pichia sp etc can use this fruit as a substrate and cause spoilage producing off flavours, odours, discolouration of the product and if contaminating microorganisms are pathogens could cause illness (Simmonete et al., 2016).However, an effective method of fruit preservation should retain the original characteristics of fruit as convenient as possible. The main methods of fruit preservation includes; Modified Atmosphere Storage (MAS), Proper washing with Potable water, Controlled Atmosphere Storage (CAS), use of preservatives, use of irradiation, use of heat, chilling and processing all of which extend the shelf life of fresh fruit produce (Dougheri et al., 2007). Chemical preservatives such as sorbate and benzoate are the preservatives which have commonly been used in preserving fruit juice by extending its shelf life (Dougheri et al., 2007; Nwachukwu and Ezeigbo, 2013) but, these preservatives have been found to have adverse effects on the health of consumers. As a result of the health risks that are associated with consistent use of chemical preservatives, the search for a more appropriate, safe and hazard-free biopreservative technique has now become the current area of interest that is being explored in the fruit and beverage industries so as to minimize the risk effects among the demanding consumers. However, antimicrobial compounds (lactic acid, diacetyl and hydrogen peroxide) produced by Lactic acid bacteria (LAB) have been proven to have great antimicrobial effect against fruit juice spoilage organisms without any adverse effect on the consumers (Mohammad, 2007). Hence, this study aims at identifying LAB isolated from fresh milk and employing it as natural biopreservative in extending the shelf life of pine apple juice.
2. Materials and Methods
- Sample collectionReplicate samples of fresh milk were obtained from Fulani herdsmen at Osogbo, Osun State, Nigeria. These were collected in sterile universal bottles and kept at temperature of 4oC before reaching the laboratory for microbial analysis. The samples were collected at different times and at different locations.Preparation and sterilization of mediaThe media used in this experiment for the isolation of LAB are De Man Rogosa Sharpe (MRS) Agar, De Man Rogosa Sharpe broth, Nutrient Agar, MacConkey Agar and Yeast Extract Agar. The media were prepared following the standard laboratory methods as described by Cheesebrough (2003).Isolation of LAB from sampleSamples were serially diluted and 1ml was taken from the sample and inoculated using pour plated method on De Man Rogosa and Sharpe (MRS) agar. The MRS agar plates were incubated for 48hrs at 35oC in anaerobic jar and subcultured severally to obtain a pure culture. Twenty pure isolates were obtained and maintained on agar slant for further characterization and identification (Bromberg et al., 2004, Oyeleke and Manga, 2008).Characterization and identification of LAB isolatesThe isolates were characterized based on colony morphology, cell morphology and biochemical tests (Cheesbrough, 2003; Oyeleke and Manga, 2008). The biochemical tests include Gram staining, catalase test, gas formation from glucose, dextran production from sucrose, starch hydrolysis etc. All strains were also tested for acid production from L- arabinose, D-xylose, galactose, D-fructose, Lactose, D-raffinose, mannitol and ducitol (Tserovska et al., 2002) and their identities confirmed using Bergey’s manual of Systematic Bacteriology(Sneath et al., 2009). Inoculum preparation and standardizationThe inoculum was prepared by aseptically inoculating a colony picked from each of the LAB streaked plates into a sterile 9ml MRS broth in test tubes using a sterile inoculating loop. The inoculated test tubes were incubated for 5 days and the antimicrobial compounds produced were checked for days 0, 3, and 5. The LAB inoculum was standardized by using 0.5 McFarland standards. McFarland standard was used as reference to adjust the turbidity of microbial suspensions so that the number of cells was equivalent to 3X103 cfu/ ml (Khunajakr et al., 2008). Determination of Lactic Acid Production by the IsolatesEstimation of lactic acid produced was determined by titration of 25ml of broth cultures of the test organisms (24hr old) with 0.1N NaOH. 3 drops of phenolphthalein were added as indicator. NaOH was then added slowly to the sample until a pink colour appeared. Each ml of 0.1N NaOH is equivalent to 90.08mg of lactic acid as stated in A.O.A.C (2000).
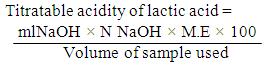 ml NaOH = Volume of NaOH usedN NaOH – Normality of NaOHM.E = Equivalenat factor = 90.08mgDetermination of Diacetyl Production by the IsolatesDiacetyl produced by the isolates was estimated by measuring 25ml of the broth cultures of the test isolates (24hrs) into conical flasks and 7.5ml hydroxylamine solution was used for residual titration. The flasks were titrated with 0.1N HCl to a green-yellow end-point using bromophenol blue as indicator. The equivalent factor of HCl to diacetyl is 21.52mg (AOAC, 2000).
ml NaOH = Volume of NaOH usedN NaOH – Normality of NaOHM.E = Equivalenat factor = 90.08mgDetermination of Diacetyl Production by the IsolatesDiacetyl produced by the isolates was estimated by measuring 25ml of the broth cultures of the test isolates (24hrs) into conical flasks and 7.5ml hydroxylamine solution was used for residual titration. The flasks were titrated with 0.1N HCl to a green-yellow end-point using bromophenol blue as indicator. The equivalent factor of HCl to diacetyl is 21.52mg (AOAC, 2000). 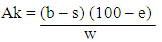 Ak = Percentage of diacetylb = Number of ml of 0.1N HCl consumed in titration of the samplee = Equivalence factor = 21.52mgw = Volume of samples = Number of ml of 0.1N HCl consumed in titration of residue sampleDetermination of Hydrogen Peroxide Production by the IsolatesAbout 20ml of diluted H2SO4 were added to 25ml of the broth cultures of the test organisms (24hrs). Titration was carried out with 0.1N KMnO4. Each ml is equivalent to 1.70mg of H2O2 and declourization of the sample was regarded as the end point (AOAC, 2000).
Ak = Percentage of diacetylb = Number of ml of 0.1N HCl consumed in titration of the samplee = Equivalence factor = 21.52mgw = Volume of samples = Number of ml of 0.1N HCl consumed in titration of residue sampleDetermination of Hydrogen Peroxide Production by the IsolatesAbout 20ml of diluted H2SO4 were added to 25ml of the broth cultures of the test organisms (24hrs). Titration was carried out with 0.1N KMnO4. Each ml is equivalent to 1.70mg of H2O2 and declourization of the sample was regarded as the end point (AOAC, 2000).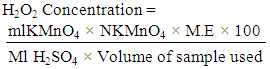 Ml KMNO4 = Volume of acid usedNKMnO4 = Normality of KMnO4Ml H2SO4 = Volume of H2SO4 addedM.E = Equivalent factor = 1.70mgLAB selection for bio preservation of pineapple juiceThe LAB isolates with high yield of antimicrobial compounds were selected for biopreservation of pineapple juice and these were Lactobacillus fermentum ML6 and Lactobacillus plantarum ML3.Preparation and extraction of pineapple juiceRipe and wholesome pineapples used were peeled, cut into pieces and blended with electric blender. The extract obtained was filtered to separate the juice from pineapple tissue. The pineapple juice was stored in a sterile container for further use (Odebunmi and Dosumu, 2003).Pasteurization of the pineapple juiceThe pine apple juice was pasteurized using High –Temperature-Short time method with water bath pasteurizer at 85oC for 15minutes in sterile McCartney bottles (Odebunmi and Dosumu, 2003).LAB selection for bio preservation of pineapple juiceThe LAB isolates with high yields of antimicrobial compounds (lactic acid, diacetyl and hydrogen peroxide) were selected for bio preservation of pineapple juice and these were Lactobacillus fermentum ML6 and Lactobacillus plantarum ML3.Biopreservative activity of LAB on pineapple juice.One millilitre of standardized inoculums of L. fermentum ML6 and L. plantarum ML3 (combined lactic cultures) was aseptically pipetted into 10ml of the pasteurized pineapple juice inside McCartney bottles and one millilitre of standardized inoculum of L. fermentum ML6 and L. plantarum ML3 (single lactic culture) was also pipetted into McCartney bottles containing 10ml of the juice samples differently. The sample bottles were stored at room temperature and monitored for a period of 7days.Monitoring of the parameters of the fruit juice(i) Physical appearance of the juiceThe physical appearance based on colour of the pineapple juice was monitored for seven days according to the method of Mohammed et al. (2013).(ii) Flavour determinationThe flavour of the juice was monitored for seven days according to the method of Mohammed et al. (2013).(iii) Microbial CountThe microbial colony count of the pine apple juice was taken for the seven days of storage and pour plate method was used. A tenfold serial dilution was carried out for both the single and combined cultures of LAB. One millilitre of the various samples were also serially diluted to 10 fold and Nutrient Agar, MacConkey Agar, Yeast Extract Agar and De man Rogosa Sharpe Agar (MRSA) were aseptically poured into the sterile Petri dishes (Cheesbrough, 2000). The Petri dishes were incubated aerobically at 35°C for 24hrs but for the MRS plates for 48hrs anaerobically. The total viable colonies were counted and recorded as colony forming unit per millilitre (cfu/ml) of the sample (Cheesbrough, 2003, Oyeleke and Manga, 2008).
Ml KMNO4 = Volume of acid usedNKMnO4 = Normality of KMnO4Ml H2SO4 = Volume of H2SO4 addedM.E = Equivalent factor = 1.70mgLAB selection for bio preservation of pineapple juiceThe LAB isolates with high yield of antimicrobial compounds were selected for biopreservation of pineapple juice and these were Lactobacillus fermentum ML6 and Lactobacillus plantarum ML3.Preparation and extraction of pineapple juiceRipe and wholesome pineapples used were peeled, cut into pieces and blended with electric blender. The extract obtained was filtered to separate the juice from pineapple tissue. The pineapple juice was stored in a sterile container for further use (Odebunmi and Dosumu, 2003).Pasteurization of the pineapple juiceThe pine apple juice was pasteurized using High –Temperature-Short time method with water bath pasteurizer at 85oC for 15minutes in sterile McCartney bottles (Odebunmi and Dosumu, 2003).LAB selection for bio preservation of pineapple juiceThe LAB isolates with high yields of antimicrobial compounds (lactic acid, diacetyl and hydrogen peroxide) were selected for bio preservation of pineapple juice and these were Lactobacillus fermentum ML6 and Lactobacillus plantarum ML3.Biopreservative activity of LAB on pineapple juice.One millilitre of standardized inoculums of L. fermentum ML6 and L. plantarum ML3 (combined lactic cultures) was aseptically pipetted into 10ml of the pasteurized pineapple juice inside McCartney bottles and one millilitre of standardized inoculum of L. fermentum ML6 and L. plantarum ML3 (single lactic culture) was also pipetted into McCartney bottles containing 10ml of the juice samples differently. The sample bottles were stored at room temperature and monitored for a period of 7days.Monitoring of the parameters of the fruit juice(i) Physical appearance of the juiceThe physical appearance based on colour of the pineapple juice was monitored for seven days according to the method of Mohammed et al. (2013).(ii) Flavour determinationThe flavour of the juice was monitored for seven days according to the method of Mohammed et al. (2013).(iii) Microbial CountThe microbial colony count of the pine apple juice was taken for the seven days of storage and pour plate method was used. A tenfold serial dilution was carried out for both the single and combined cultures of LAB. One millilitre of the various samples were also serially diluted to 10 fold and Nutrient Agar, MacConkey Agar, Yeast Extract Agar and De man Rogosa Sharpe Agar (MRSA) were aseptically poured into the sterile Petri dishes (Cheesbrough, 2000). The Petri dishes were incubated aerobically at 35°C for 24hrs but for the MRS plates for 48hrs anaerobically. The total viable colonies were counted and recorded as colony forming unit per millilitre (cfu/ml) of the sample (Cheesbrough, 2003, Oyeleke and Manga, 2008). 3. Results
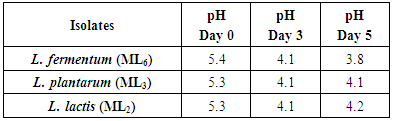
- Twenty LAB isolates were obtained from the milk samples and were characterized. They were identified as L. fermentum, L. plantarum and L. lactis respectively. The isolate L. fermentum has the highest rate of occurrence of 60%, L. plantarum 20% and L. lactis 20%. The pH of the lactic cultures was determined on 5 days of growth and the result is presented in Table 1. L. fermentum had the lowest pH, followed by L. plantarum and L. lactis (3.8, 4.1, 4.2) respectively.
|
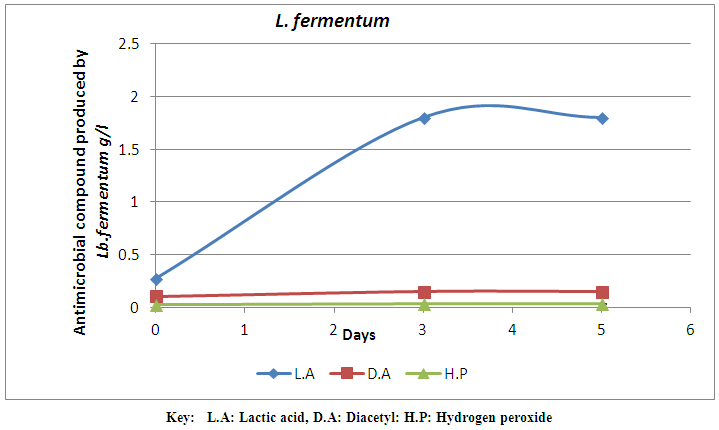 | Figure 1. Antimicrobial compounds produced by L. fermentum monitored for 7 days |
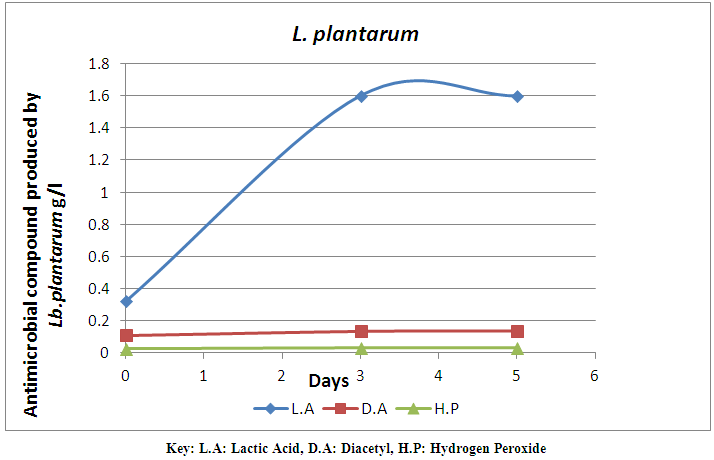 | Figure 2. Antimicrobial compound produced by L. plantarum monitored for 7 days |
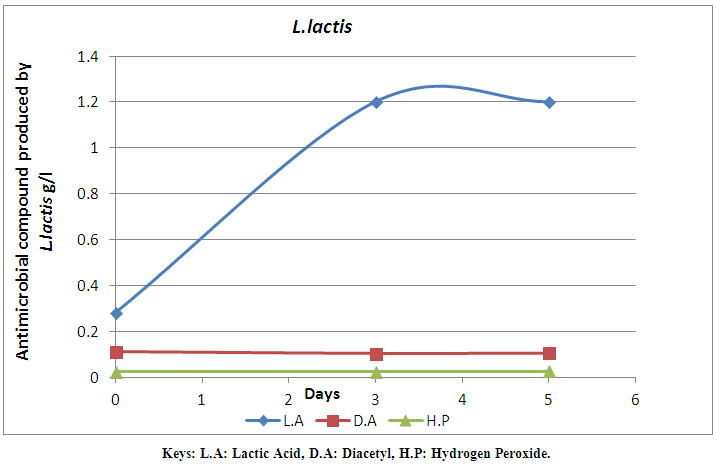 | Figure 3. Antimicrobial compound production by L. lactis monitored for 7 days |
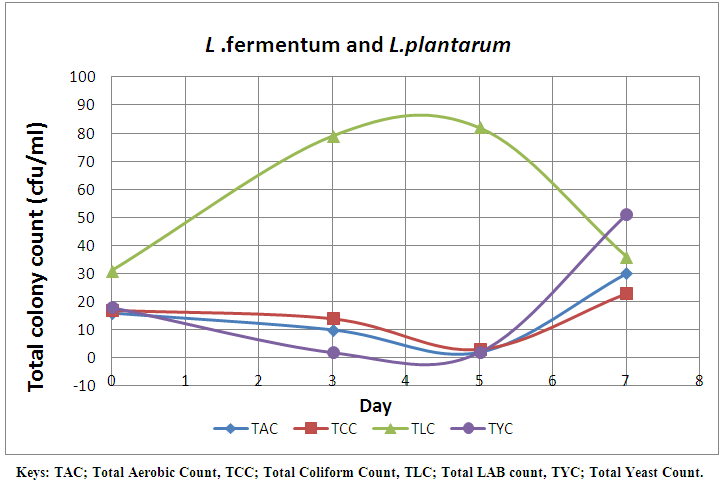 | Figure 4. Effect of combined cultures of L. fermentum and L. plantarum on the microbial load of pineapple juice monitored for 7 days |
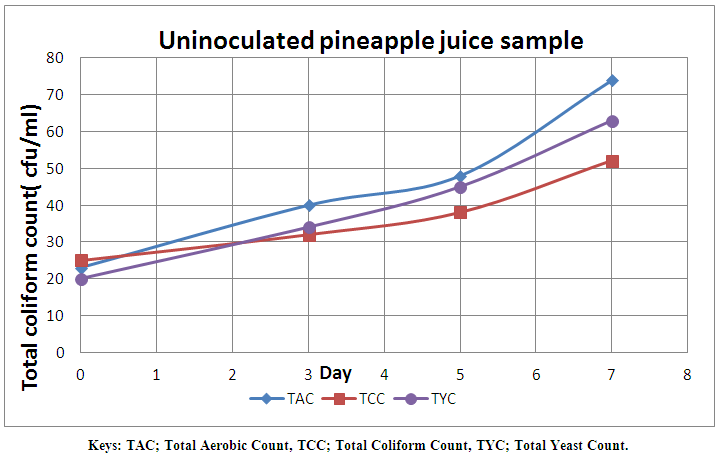 | Figure 5. Microbial load of uninoculated pineapple juice without Lactic cultures monitored for seven days |
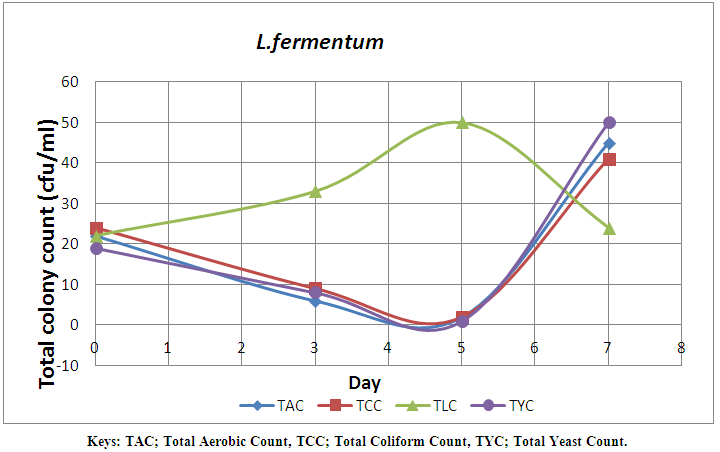 | Figure 6. Effect of single starter of L. fermentum on the microbial load of pineapple juice monitored for seven days |
4. Discussion
- A total number of 20 strains of LAB were isolated from milk samples in this study. The LAB strains were characterized and identified. The identified LAB were grouped into two. The first group is the Lactobacilli group that was represented by two species, L. fermentum (60%) and L. plantarum (20%). The cocci group is the second group that was identified as L. lactis (20%). The identification by classification into groups was in accordance with the method used by Tserovska et al. (2002) and Morek (2011). The lower number of lactic acid cocci is probably due to their inability to compete with lactic acid bacilli in mixed cultures (Togo et al., 2002). The morphological, biochemical and physiological characterization of the isolates revealed that the isolates that produced lactic acid in abundance belong to the Lactobacilli group which are L. fermentum and L. plantarum. The variation of acidification was monitored for all the isolates. The decrease in the pH of lactic cultures after 48hr showed the lactic acid production efficiency of the isolates. Lactic acid bacteria are known to produce antimicrobial substances mainly in the form of organic acids and other metabolites (Tserovska et al., 2002; Obadina et al., 2006). The LAB isolated from milk in this study produced different antimicrobial compounds and the quantity varies with time. The increase in the production of lactic acid with time has been attributed to lower pH which permits the growth of LAB. The LAB strain of L. fermentum isolated from the milk sample has the highest production of antimicrobial compounds on day 5. The production of antimicrobial compounds by these LAB strains confers the potential ability on them to extend the shelf life of fruit juice and reduce the microbial load. Antimicrobial compounds can be applied to foods either as purified chemical agents, or as viable cultures in the case of fermented products (O’Sullivan et al., 2002). The inoculation of lactic cultures of L. fermentum ML6 and L. plantarum ML3 into pineapple juice revealed that pH, storage temperature and microbial load played significant roles in shelf life determination. This agrees with the report of FSAI (2011) that the shelf life of many food products is dependent on storage temperature and microbial load. At room storage temperature (23±10°C), the pineapple juice was preserved and this is probably due to the presence of lactic cultures which grows optimally at 30±5°C and produce metabolites like lactic acid, diacetyl, hydrogen peroxide and bacteriocin which probably inhibited the growth of spoilage and pathogenic microorganisms in the pineapple juice under study. This is similar to the findings of Ogunbanwo et al. (2003) and FSAI (2011) who reported that LAB grows optimally at 30-37°C and produce metabolites like bacteriocin which probably inhibits the growth of spoilage and pathogenic microorganisms such as S. aureus and other microflora in the pineapple juice. Tserovska et al. (2002) also reported that (LAB) grow optimally at pH 5.8 to 6.5 and produce metabolites like lactic acid and bacteriocin (bio preservative) which are active against food borne pathogens. Similar observations were made in this study where lactic cultures inoculated into pineapple juice grew maximally and produced high amount of antimicrobial compounds at pH 3.8 and 4.0. The lactic cultures (L. fermentum ML6 and L. plantarum ML3) inoculated into pineapple juice proved effective in reducing the microbial load, and hence spoilage of the product and extended the shelf life of pineapple juice at room temperature for 5 days. The shelf life extension of pine apple juice could be as a result of increased antimicrobial activity of the lactic cultures and hence biopreservation. This is in correlation with the report of O’Sullivan et al. (2002) and Tserovska et al. (2002) who opined that; as an alternative to using antimicrobial compounds for bio preservation of foods, direct introduction of live antimicrobial-producing culture of LAB as a protective starter could help in achieving favourable results in some food systems. This contribution is due to competition for nutrients and the presence of inhibitor agents produced, including organic acids, hydrogen peroxide, and bacteriocins. The antimicrobial compounds produced by LAB obtained from milk may be used to solve the problem of growth of contaminating microorganisms during processing in the food industry. The use of lactic cultures with biopreservative activity could improve the quality of food and increase its safety by inhibiting the food-borne pathogens and spoilage microorganisms.
5. Conclusions
- The growth of pathogenic and spoilage microorganisms in pineapple juice under study were inhibited by L. fermentum and L. plantarum. LAB are very important in that their presence in food can enhance the extension of the shelf life of the food products and reduce the microbial load. Therefore, the presence of antimicrobial compounds producing LAB in juices and fermented foods can enhance the safety and shelf life extension of the food products and will also serve as alternative to chemical preservatives or additives in food preservation.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML