-
Paper Information
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Frontiers in Science
p-ISSN: 2166-6083 e-ISSN: 2166-6113
2013; 3(4): 107-113
doi:10.5923/j.fs.20130304.01
Classification of Ovarian Stages of Alestes baremoze (Joannis, 1835): A Step towards Understanding Its Reproductive Biology
N. Kasozi1, G. I. Degu1, K. Atibuni1, M. Kisekka2, A. Owori-Wadunde3, S. Mugerwa4
1Livestock and Fisheries programme, Abi Zonal Agricultural Research and Development Institute, P.O. Box 219, Arua-Uganda
2Department of Veterinary Pharmacy, Clinics and Comparative Medicine-Makerere University: P.O Box 7062 Kampala-Uganda
3Aquaculture Research and Development Center (ARDC), Kajjansi. P. O. Box 530 Kampala-Uganda
4National Livestock Resources Research Institute, P. O. Box 96, Tororo-Uganda
Correspondence to: N. Kasozi, Livestock and Fisheries programme, Abi Zonal Agricultural Research and Development Institute, P.O. Box 219, Arua-Uganda.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The indigenous Alestes baremoze is one of the fishes endemic to Lake Albert. In this study, the maturity stages of ovaries from 100 A.baremoze fish collected from Lake Albert were determined. Maturity condition was assessed by macroscopic inspection of gonads and histological methods. Macroscopic inspection was based on alterations in ovary size and appearance, whereas histological methods evaluated changes in oocyte stages. Gonado-somatic index (GSI) was used to assess the reproductive seasonality for a period of twelve months and was calculated as the proportion of ovary to body weight. Histological analysis confirmed the overall general appropriateness of macroscopic staging with stage IV being the most abundant in the samples and still the highest matching proportion between the two classifications. A correspondence graph obtained reveals few challenges in matching between macroscopic and microscopic classification for stages II and VI. Among ovaries macroscopically classified, 1% immature, 20% developing, 70% at spawning stage and 9% were at spent stage. The spawning pattern exhibited by A. baremoze indicate that this species undergo short spawning periods. This information is important in estimating maturity status which is a key in regulating A. baremoze fishery basing on spawning biomass.
Keywords: Alestes baremoze, Histology macroscopic, Ovary
Cite this paper: N. Kasozi, G. I. Degu, K. Atibuni, M. Kisekka, A. Owori-Wadunde, S. Mugerwa, Classification of Ovarian Stages of Alestes baremoze (Joannis, 1835): A Step towards Understanding Its Reproductive Biology, Frontiers in Science, Vol. 3 No. 4, 2013, pp. 107-113. doi: 10.5923/j.fs.20130304.01.
Article Outline
1. Introduction
- Alestes baremoze is native to freshwater systems in Africa, thriving well in both lacustrine and riverine conditions[1]. In particular, A. baremoze is widely distributed along the River Nile, including the Delta Lakes, Rashid branch and Lower Nile. However, the survival and proliferation of the species is constrained by several factors including pollution, agricultural development leading to habitat loss and degradation, overfishing and water abstraction[2]. As such, the North Africa regional assessment considers the species regionally extinct. In Uganda, the species is found within Lake Albert, the Albert and Murchison Niles and it’s a delicacy for Northern and West-Nile tribes[3]. It is one of the most commercially important species in L. Albert however its biology is imperfectly known as there has been a tendency to deal with other common species such as Labeo victorianus [4]. Alestes fish populations have been subjected to climatic changes and fishing pressure since the early 1950s. This has resulted in declining fish population trends. Today, small pelagic fishes such as Brycinus nurse and Neobola bredoi contribute to almost 80% of the fish catches on Lake Albert. The current data contrasts with the historical catch data which ranked Alestes spp among the dominant commercial fish between 1969 and 1990[5]. Information on classification of ovaries for A. baremoze is still largely unknown with most of the previous studies focusing on population densities[6] and species responses to climatic variability and change[5]. Limited efforts have been devoted to classify the ovaries or elucidate the reproductive seasonality of A. baremoze. Yet knowledge contributing to correct classification of ovaries into developmental stages is fundamental in estimating maturity status, a prerequisite for setting annual catch quotas using a harvest rate strategy based on spawning biomass estimates[7]. In addition, the identification and classification of maturity stages aids the determination of spawning period according to different geographical and environmental areas and understanding the relationship between length at maturity and fishery exploitation[8].The study therefore sought to contribute to understanding of the species’ reproductive biology by comparing GSI as well as determining the maturity stages of A. baremoze over twelve months period of study.
2. Material and Methods
2.1. Study Area
- The study was conducted at Abok landing site (02° 14.46‘N 31°19.15‘E) located in Panyamur Sub County on Lake Albert (Figure 1). The lake is the second largest fishery in Uganda, after Lake Victoria and it is among the few that supports a diverse population of native fish species including Alestes baremoze. The lake is shared by two countries Uganda and Democratic Republic of Congo. In Uganda, it is shared by riparian districts namely; Ntoroko, Kibale, Hoima, Buliisa and Nebbi. It covers a total estimated surface area of 5,270km2 with approximately 60% within Uganda waters[9].
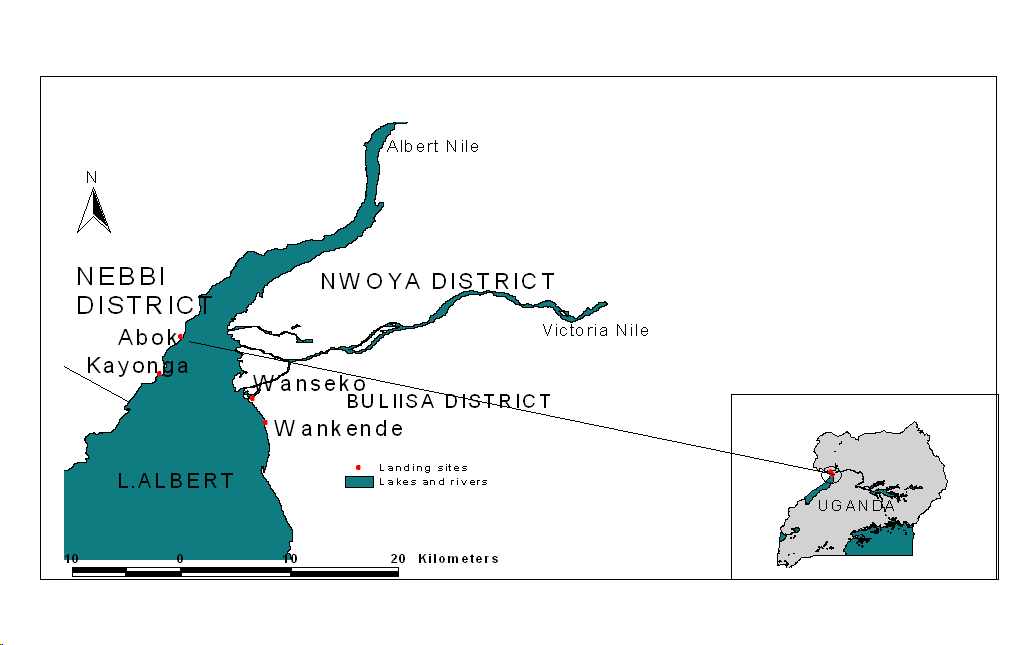 | Figure 1. Map of Uganda with an insert of Lake Albert showing the location of Abok landing site |
2.2. Fish Sampling and Measurement
- The local fishermen collected A. baremoze using monofilaments of various stretched mesh sizes (3 to 4 inches) between November 2012 and October 2013. A total of 244 female fish were collected during this period and for each specimen, measurements for ovary weight and total mass (W) were taken and used for assessing reproductive seasonality. Spawning seasonality was assessed by Gonadosomatic index as described by[10; 11]. It was calculated as;
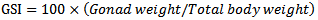 | (1) |
2.3. Maturity Stage Samples
- Only 100 ovaries were sampled and classified to maturity stages based on macroscopic and microscopic characteristics. A sample of the ovary was taken from the sampled fish then fixed and preserved in Bouin’s fixative. The samples were then processed using standard histological techniques as described by[12] and involved (i) fixation; (ii) infiltration; (iii) sectioning; (iv) mounting; (v) staining. Histological analysis was performed to confirm the accuracy of macroscopic determinations of maturity. Oocytes were photographed at each developmental stage and were classified into developmental stages.
3. Results
3.1. Maturity Stages
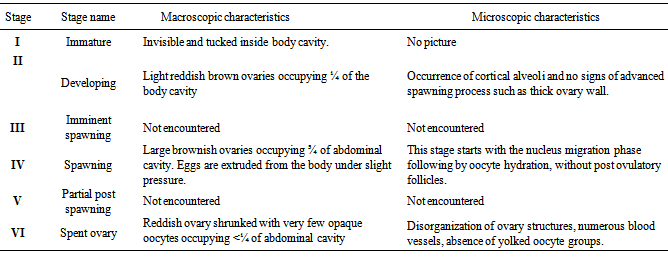
- The maturity stage for each fish was determined using the six-degree macroscopic maturity scale shown in Table 1[13]; modified for six stages;[14]. This six stage scale includes: I = immature, II = developing, III = imminent spawning, IV = spawning, V= partial post spawning and VI = spent. This type of scale seems to be more discriminate and less subject to interpretative error compared to others. The most advanced oocyte stage present and ovary coloration varied with developmental stage. Ovaries with their most advanced oocytes at peri-nucleus or yolk vesicle stages were brown (Figure 2). Stages III and V were not encountered in the study. However, for histological examination only three stages were compared as the structures for immature fish were tucked inside the fish’s body cavities (Figures 3, 4 and 5). The macroscopic comparisons revealed that stage I was not widely represented in the samples (around 1%). Stage II was only represented by 20% while stage IV (hydrated) was the most recognizable by its evident attributes as oocytes could be easily seen within the translucent ovarian tissue. Only 9% of the ovaries were categories in stage VI.
|
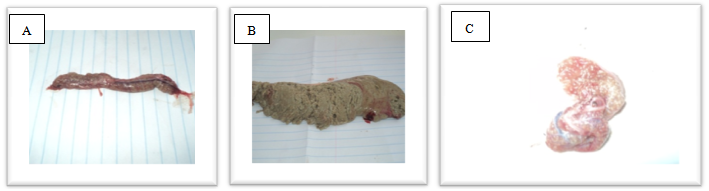 | Figure 2. Three macroscopic maturity stages of ovary. (A) Developing ovary; (B) Spawning and (C) Spent ovary |
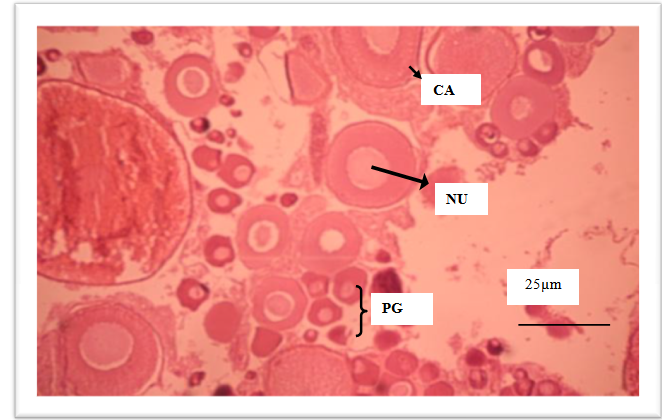 | Figure 3. Developing ovary with oocytes in nucleus (NU) migration. Ovary with many primary growth oocytes PG – Primary growth oocytes; CA – cortical alveolar oocytes |
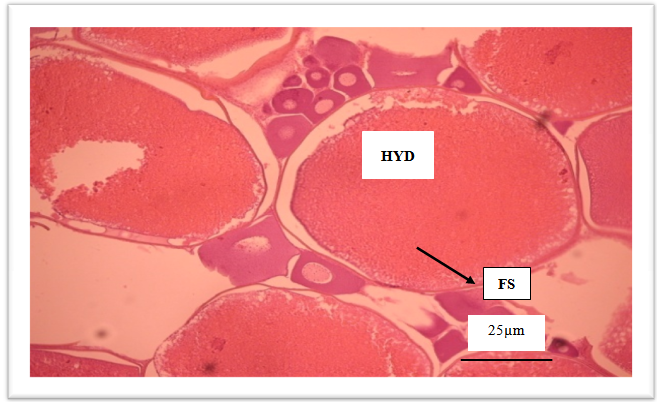 | Figure 4. Spawning ovary with hydrated oocytes (HYD) and fused yolk globules (FS) |
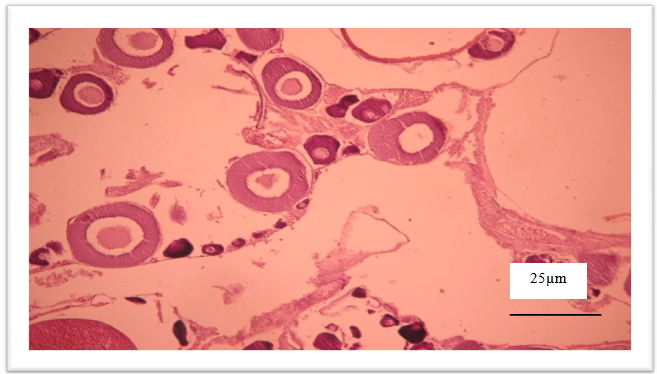 | Figure 5. Spent ovary with disorganization of ovary structures |
3.2. Macroscopic and Microscopic Correspondences
- The abundance and percentage of correspondence was highest for stage IV (100%) followed by stage VI (77%) (Figure 6). The percentage of correct classification for stage II was slightly low 60% compared to other stages.
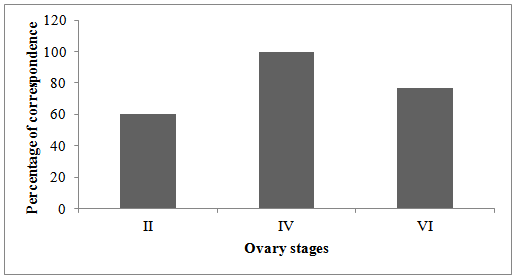 | Figure 6. Percentage correspondence between macroscopic and microscopic classifications |
3.3. Reproductive Seasonality
- Female gonadosomatic indices varied across the twelve months of the study (Figure 7). Minimum observed GSI occurred in December -2012 (2.1 ± 2.0%), May (2.4± June (2.3±1.7%), July (2.5±1.7%) and March 2013 (2.7 ± 1.6%). The maximum GSI value of (5.4±1.8%) was reached in January-2013 followed closely with (5.3±1.8%) of October.
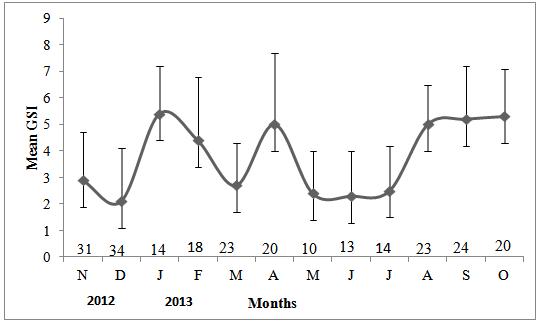 | Figure 7. Variation in mean monthly female GSI over the study period (±standard deviation). Numbers along the x-axis show sample size for each month |
4. Discussion
- A. baremoze also presents challenges, as for many other species in distinguishing developing and spent females macroscopically[15; 14]. In both stages the oocytes are not easily visible. This challenge has an impact on the estimation of the mature proportion of the stock since resting females have already contributed to the spawning biomass of that year and are macroscopically considered immature. Stage IV was the most abundant in the samples and showed the highest matching proportion between micro- and macroscopic classifications. This is because this stage was generally easily recognizable by visual inspection of fresh individuals and also contains the bulk of ovaries. Spawning in fish is mainly described as total and partial spawners[13]. Total spawners consist of eggs which are going to be spawned by the individual fish in a single breeding period to develop synchronously. Fish that undergo partial spawning consist of eggs which ripen at very different stages of development and can be found at any one time in the same ovary both before and during spawning. Basing on the observable characteristics during the maturity stages, A. baremoze undergo total spawning as no opaque eggs were noticed to be left in the ovary during the spawning stage. Female length at maturity was not estimated because the sampling was got from the fishermen at the landing and the distribution of maturity stages at Abok landing may not be representative of the distribution of sexual maturity within the population in the Lake Albert.Temporal changes in GSI have been used for estimating spawning periods of Labeo victorianus[4] and Puntius schwanenfeldii[16]. The spawning pattern exhibited by A.baremoze across the twelve months of study indicate that this species undergo short spawning periods. The longer period exhibited by this fish lasted for only three months (August - September) followed by two months (January – February) and another lasted by one month (April). This is an indication that the lake is facing multiple environmental changes, including declining fish species diversity, hyper-eutrophication, hypoxia, and reduced fish catches. It is known that environmental conditions play an important role in influencing fish reproduction by stimulating sensory organs that induce the production of gonad hormones, which can induce physiological or behavioral responses[17]. Basing on the rapid changes of the GSI, reproduction of Alestes baremoze may not follow a synchronized spawning that’s influenced by rainy season but rather by photoperiod, water temperature, water current and cycles of the moon. A deviation from rainy season synchronized spawning has been previously reported in Brycinus lateralis belonging Alestidae family.[18] noted that spawning of Brycinus lateralis in the Okavango Delta was not necessarily flood dependent. A deviation from the typical flood spawning pattern has also been reported in the Sio River for Labeo victorianus[4]. Fish species that are flood dependent tend to be highly mobile, moving around the floodplain to reproduce and forage. The species that are flood independent are relatively resident and due to their reproductive pattern, would gain little from large scale movements. A. baremoze likely to fall in the latter category, with spawning activity correlated with the changes in cycles of the moon, water temperature and wind rather than the flood cycle. Lake Albert remains one of the few lakes in the country supporting a multi-species fishery. The legal minimum gill net mesh size on this lake is set at 5 inches[3]. However, the emerging fisheries comprise mostly pelagic fishes that grow to a very small adult size and therefore cannot be fished with the set gill net mesh size. The emergence of the light fishery is a response to the removal through intense fishing of large sized fishes such as A. baremoze over years[5]. This high exploitation rates have led to reduction in biological parameters especially the size at which they mature. A.baremoze has been estimated to mature at17 cm fork length compared to the historical size of 27cm[3]. This is further evidenced by the high number of fishes within the spawning stage recorded yet the fishermen applied a 2-3 inch mesh sizes during their fishing operations. Therefore the legal 5 inch mesh set by the government on this lake may not apply. A 2½ inch net can catch mature spawning A. baremoze therefore these fishes need their own minimum mesh regulation for effective exploitation as the legalized nets of mesh size 5 inches and over may not apply.
5. Conclusions
- In conclusion, factors which result into discrepancies between macroscopic and histological observations such research subjectivity; time of sampling; the level of fish injury due to the fishing operations; and time between the catch to examination of the samples need to be put into consideration. Further research is required to understand how environmental conditions such as water current, water level, rainfall, wind affects the reproductive biology of A. baremoze in Lake Albert.
ACKNOWLEDGEMENTS
- We would like to thank Faustino the Fisheries Officer –Panyamur Landing site who assisted us during the field sampling and all fishermen of Abok fishing village. The authors wish to thank Dr. Julius Mukalazi for the support extended during the study. We are also grateful to Agricultural Technology and Agribusiness Advisory Services (ATAAS) project for funding the study.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-text HTML
Full-text HTML