-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Frontiers in Science
p-ISSN: 2166-6083 e-ISSN: 2166-6113
2012; 2(6): 221-225
doi: 10.5923/j.fs.20120206.15
Effects of Hibiscus Sabdariffa Extracts on 2, 4-Dinitrophenylhydrazine-induced Cytotoxicity in Rabbits
Augustine O. Olusola1, Adesayo O. Olusola1, Sunday O. Bada1, Fredrick O. Obi2
1Department of Biochemistry, Faculty of Science, Adekunle Ajasin University, Akungba Akoko, Ondo State, Nigeria
2Department of Biochemistry, Faculty of Life Sciences, University of Benin, Benin City, Edo State, Nigeria
Correspondence to: Augustine O. Olusola, Department of Biochemistry, Faculty of Science, Adekunle Ajasin University, Akungba Akoko, Ondo State, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
This study examines the effects of aqueous and anthocyanin-rich extracts of Hibiscus sabdariffa calyces on 2,4-dinitrophenylhydrazine (DNPH)-induced biochemical changes in the blood, brain and liver of rabbits. Changes in tissue levels of glucose and protein were used as indices of alteration and protection. Thirty male adult rabbits used for the study were divided into six groups. Group 1, the control took only water while animals in groups 2,3,5 and 6 received 100 mg/kg body weight of the extracts once daily for 28 days. After 22nd day of treatment, the rabbits in groups 4, 5 and 6 received 28 mg/kg body weight of DNPH for the remaining 5 days of treatment, after which the animals were sacrificed. Relative to control, animals treated with DNPH alone (Group 4) showed significant (p<0.05) reduction in the levels of glucose and protein in the tissues. However, animals treated with each of the aqueous extract and anthocyanin-rich extract prior to DNPH administration (Groups 5 and 6), showed no significant alteration in the tissue levels of these parameters. Our findings show that extracts of the Hibiscus sabdariffa contain phytochemicals that effectively ameliorate 2, 4-dinitrophenylhydrazine-induced cytotoxicity in rabbits.
Keywords: Hibiscus Sabdariffa Calyces, Aqueous, Anthocyanin-Rich Extracts, Glucose, Protein
Cite this paper: Augustine O. Olusola, Adesayo O. Olusola, Sunday O. Bada, Fredrick O. Obi, "Effects of Hibiscus Sabdariffa Extracts on 2, 4-Dinitrophenylhydrazine-induced Cytotoxicity in Rabbits", Frontiers in Science, Vol. 2 No. 6, 2012, pp. 221-225. doi: 10.5923/j.fs.20120206.15.
Article Outline
1. Introduction
- Hibiscus sabdariffa Linn (Roselle) belongs to the family of Malvaceae, which is native to old World tropics, probably in the East Indies; now cultivated throughout the tropics[2]. The vegetable is widely grown and commonly used as port herb or soup in the northern part of Nigeria. In Nigeria especially in the northern part, the extract of the red calyces is consumed as a beverage known as zobo.Ethnobotanical information regarding Hibiscus sabdariffa reveals the following medicinal uses: diuretic, diaphoretic, antibacterial agent, antifungal agent, mild laxative, sedative, antihypertensive, gastrointestinal disorder treatment, hypercholesterolemia treatment, kidney stone treatment, liver damage treatment, agent for decreasing the viscosity of the blood, and agent for treating the after effects of drunkenness[1, 3 and 14]. Among the chemical constituents of the flower are the flavonoids, gossypetine, hibiscetine, anthocyanin and sabdaretine[18]. Certain amounts of delphinidin-3-monoglucoside andcyaniding-3-monoglucoside which constitute the anthocyanins are also present[10].Some studies have reported that Hibiscus sabdariffa is effective for decreasing the levels of total lipids, cholesterol and triacylglycerol, suggesting the possibility that Hibiscus sabdariffa functions as hypolipidemic agent[3]. Studies on the effect of Hibiscus sabdariffa calyx extract on the protein and glucose levels are however, scanty. The present study is therefore aimed at evaluating the effect of Hibiscus sabdariffa extracts on 2,4-dinitrophenylhydrazine-induced changes in glucose and protein levels in the tissues of rabbits.Phenylhydrazine and its derivatives 2,4-dinitrophenylhydrazine are toxic agents. Their toxic action has been attributed to their ability to undergo auto oxidation. This increased oxidant potential enables them to oxidize enzymes, membrane protein and hemoglobin. Phenylhydrazine is able to initiate lipid peroxidation in membrane phospholipids[5] while 2,4-dinitrophenylhydrazine has been shown to be capable of inducing lipid peroxidation and other oxidative damage in rabbits[15,16 and 17] and rats[12].The ability of 2,4-DNPH to induce lipid peroxidation and other free radical damage makes it an appropriate model toxicant for testing the claim that the extract of Hibiscus sabdariffa Linn calyces can protect tissues from oxidative stress-induced changes and other attendant biochemical changes.
2. Materials and Methods
2.1. Plant Material
- Fresh calyces of H. sabdariffa were harvested from Botanical Gardens University of Ilorin, Kwara State, Nigeria. They were dried under continuous air-flow maintained at 25℃ until constant weight. Identification and taxonomical classifications were done at herbarium of the Department of Plant Biology and Biotechnology, University of Benin, Benin City, Edo-State, Nigeria.
2.2. Animals
- Thirty (30) rabbits (Oryctolagus cuniculus) used for this research work were obtained from a private breeder in Benin City. The animals weighed 800-1000 g on purchase and were in very good state of health as confirmed by a veterinary physician. The animals were housed in twos (same sex) in improvised rabbit cages composed of wire mesh (100cmX40cmX30cm) under 14 hr/10 hr light/dark regimen. They were fed with growers mash (obtained from Bendel Flours and Feed Mill, Ewu, Edo State, Nigeria) and water ad libitum. The animals were protected from parasite infestation by proper veterinary management throughout the duration of the treatment.
2.3. Preparation of Aqueous Extract
- One hundred grams of dried calyces of Hibiscus sabdariffa were soaked in one litre of distilled water for 12 hours to obtain a red coloured extract.
2.4. Preparation of Anthocyanin-rich Extract from Plant Materials
- Anthocyanin extract from Hibiscus sabdariffa calyces was prepared according to the method described by[4]. One kilogram of Hibiscus sabdariffa calyces was pulverized by means of Binatone blender and extracted with 10 litres of 0.1 % trifluoroacetic acid (TFA) aqueous solution for 12 hours at 20℃ on an orbital shaker. The extract was filtered through filter paper (Advantech number 5C). A portion of the filtrate (10 ml) was applied to sepabeads SP-207 resin column (Mitsubishi Chemicals, Japan). The resin was washed with 3 litres of water and then eluted with 50 % ethanol solution containing 0.1 % TFA. The eluate was dried under vacuum at 20 ℃ and then freeze-dried. The dried sample obtained was resuspended in distilled water and kept in the refrigerator until required for oral administration and biochemical investigation.
2.5. Experimental Design
- Thirty (30) rabbits weighing 800-1000 g were used for this research work. They were randomly selected into six (6) experimental groups as shown below. The experiment lasted for 28 days.Group 1: Water treated control. Each rabbit was given distilled water, 2.5 ml/kg body weight.Group 2: Aqueous extract of H. sabdariffa was administered at a dose of 100 mg/kg body weight, to each rabbit in this group by gavage.Group 3: Anthocyanin-rich extract of H. sabdariffa was administered at a dose of 100 mg/kg body weight, to each rabbit in this group by gavage.Group 4: 2, 4-Dinitrophenylhydrazine was administered at a dose of 28 mg/kg body weight intraperitoneally to each rabbit in this group during the last 5 days of the 28-day study period before sacrifice. Group 5: Aqueous extract of H. sabdariffa was administered at a dose of 100 mg/kg body weight for 28 days to each rabbit in this group accompanied with 28 mg/kg body weight of 2, 4-dinitrophenylhydrazine administered intraperitoneally daily from day 24 (5 days 2,4-DNPH treated) before sacrifice. Group 6: Anthocyanin-rich extract of H. sabdariffa was administered at a dose of 100 mg/kg body weight for 28 days to each rabbit in this group accompanied with 28 mg/kg body weight of 2, 4-dinitrophenylhydrazine administered intraperitoneally daily from day 24 (5 days 2,4-DNPH treated) before sacrifice.
2.6. Biochemical Determinations
- Tissue glucose and protein were determined using standard assay kits from Randox.
2.7. Statistical Analysis
- The data obtained were subjected to standard statistical analysis of variance (ANOVA) using the SAS software[19]. Treatment means were compared using the Duncan procedure of the same software. The significance level was set at P<0.05.
3. Results
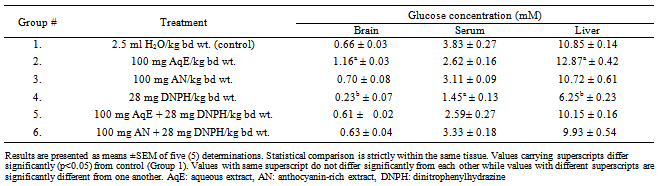
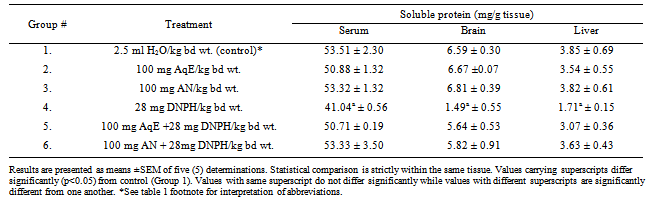
- Table 1 shows the effect of DNPH, aqueous extract of HS and HS anthocyanins on glucose levels in the brain, serum and liver. The data show that treatment of rabbits with the DNPH significantly (p<0.05) reduced glucose levels in the brain, serum and liver (Group 4) relative to control (Group 1). Treatment of rabbits with aqueous extract of HS significantly (p<0.05) increased glucose levels in the brain and liver relative to control (Group 1). Treatment with the anthocyanin-rich extract did not alter glucose level in the brain, serum and the liver. Furthermore, prior treatment with the aqueous extract of HS and the anthocyanin-rich extract before DNPH administration (Groups 5 and 6) prevented DNPH-induced glucose reduction in brain, serum and liver. The effects of DNPH, aqueous extract of HS and HS anthocyanins on the levels of soluble protein in the serum, brain and liver of rabbits is presented in Table 2. Treatment with DNPH significantly (p<0.05) reduced the soluble protein levels in the serum, brain and liver of rabbits compared to control. Rabbits that received each of the extracts before DNPH administration (Groups 5 and 6) did not show any significant reduction in the levels of tissue soluble proteins when compared with the control group.
|
|
4. Discussion
4.1. Tissue Glucose
- There is an increasing body of research evidence that suggest that although anthocyanins protect tissues against oxidative damage, they may also be denying cells of essential nutrients such as glucose. To verify this claim, there is need to assess the level of glucose in selected tissues when anthocyanin is administered. The high glucose level recorded for the group exposed to aqueous extract of H. sabdariffa may be due to the carbohydrate content of the extract which is a known component of anthocyanin. However, anthocyanin extract administration did not significantly increase glucose level in rabbit brain homogenate. The inability of the anthocyanin to significantly increase brain glucose level is due to (1) The purification process which allows for exclusion of glucose residues that could have accounted for the high glucose concentration in brain homogenate in the whole extract group. (2) During digestion, the anthocyanin sugar moieties are known to competitively inhibit glucose transporters. (3) Anthocyanidins are major phytochemicals known to inhibit alpha-amylase; the enzyme which catalyses the digestion of polysaccharides prior to absorption. (4) Ascorbate may have a similar effect on the intestinal absorption of glucose as do anthocyanins, due to structural similarity between glucose and ascorbate molecules.The significant reduction in the glucose level of brain homogenate of DNPH treated group when compared with control may be partly due to the need for detoxification of this molecule by the liver and its excretion in form of glycosides, thereby placing pressure on the liver glycogen reserve. In starvation stage, there is little or no glycogen store capable of supplying the needed glucose and since the brain is highly selective of glucose for metabolism, the effect is felt as reduced glucose level in the brain. Also, the interaction of the DNPH with intestinal epithelial cells could lead to the destruction of the cells thereby reducing their glucose-absorptive functions. Treatment with aqueous extract of H. sabdariffa and anthocyanin extract significantly restored glucose level in the brain. The serum represents the reservoir of glucose available for utilization by peripheral tissues, storage by kidney, liver and muscles and medium for excretion in case of hyperglycemia. The aqueous extract of H. sabdariffa and anthocyanin extract, showed a decrease in the serum glucose level .This result either shows the ability of anthocyanins to reduce both the digestion and absorption of dietary carbohydrates or increase the tissue assimilation or renal clearance of glucose. The significant decrease in the serum glucose level of DNPH-treated group when compared with the control is possibly due to a complex interplay of various factors: there is a possibility of the lipoperoxidative destruction of the parietal cells of the small intestine indicating gastro-intestinal toxicity. Since the cells in these tissues are primarily involved in digestion and absorption, their destruction will also reduce carbohydrate digestion and absorption. Another reasonable explanation for this finding is the detoxifying function of the liver. The presence of xenobiotic agent in the liver induces the synthesis and secretion of xenobiotic metabolizing enzyme-cytochrome P-450 monooxygenase which utilizes NADPH and the reduced xenobiotic agent. The reaction proceeds with the oxidation of NADPH to NADP+, the sustenance of the detoxification process is ensured by the reduction of NADP+, a reaction which involves oxidation of glucose-6-phosphate (a phosphorylation reaction product of glucose). After hydroxylation reaction of cytochrome P-450 monooxygenase, the partially polar compounds formed are then made more polar to aid excretion. This reaction proceeds in the presence of UDP-glucuronide (a product derived from glucose via oxidation). The glucose molecules needed for this vast array of reactions is derived either from diet or those stored in form of glycogen which are ultimately derived from the dietary carbohydrates. So in the presence of reduced carbohydrate digestion and absorption; and or increased glycogen utilization for xenobiotic metabolism and tissue uptake of glucose from the blood, the decrease in the serum level of glucose in the presence of DNPH is then biochemically justified. By reverting the factors explained above, aqueous extract of H. sabdariffa and anthocyanin extract appeared to restore the serum glucose levels under condition of their pretreatment prior to DNPH administration. The liver is metabolically active and diverse. Its metabolism revolves majorly around carbohydrate and its metabolites. Consequently, the level of glucose in the liver cells is not only good indication of its metabolic state but its function as an organ. The significantly increased glucose level in the liver of rabbits treated with aqueous extract of H. sabdariffa when compared with the control is most likely due to the proximate composition of the extract, H. sabdariffa is known to contain carbohydrates other than those which are known components of the glycoside derivatives of anthocyanidin (aglycone). To explain the significant reduction in the glucose level of the liver after treatment with DNPH, DNPH must be viewed as a xenobiotic agent that must be detoxified so as to prevent its accumulation and subsequently resulting in hepatotoxicity. The presence of xenobiotic agent in the liver induces the xenobiotic metabolizing enzyme-cytochrome P-450 monooxygenase which utilizes NADPH and the reduced form of xenobiotic agent. The reaction proceeds with the oxidation of NADPH to NADP+, the sustenance of the detoxification process is ensured by the reduction of NADP+, a reaction which involves oxidation of glucose-6-phosphate (a phosphorylation reaction product of glucose). After hydroxylation reaction of cytochrome P-450 monooxygenase, the partially polar compound formed are then made more polar to aid excretion. This reaction proceeds in the presence of UDP-glucuronide (a product derived from glucose via oxidation), therefore, resulting in decreased glucose level.
4.2. Total Soluble Protein
- The results show that each of the aqueous extract of H. sabdariffa and anthocyanin isolate has the individual potential to counteract the soluble protein depletion induced by oxidative stress, in all the tissues. This finding totally agrees with the reports of[9, 13 and 22]. Also, in the three tissues, anthocyanin extract appeared to be more potent in this capacity. The biochemical basis underlying the ability of anthocyanin to potentially increase the expression of soluble protein in most cells has not been fully elucidated[21]. The experimental group treated with DNPH exhibited reduced soluble protein levels in all the tissues under investigation. The decrease in protein content of the liver and brain homogenate can be attributed to the increased cell membrane disruption and subsequent loss of protein content of cytoplasmic milieu, increased protein degradation in the cell as a result of changes in the pH and chemical modification of intracellular protein and oxidative damage of the cell membrane and subsequent necrosis of the tissues. The mechanism of cytotoxicity of DNPH has also been characterized as similar to some aromatic ring-structured drugs and CCl4 induced tissue necrosis[6,7]. The reduced protein content of the serum must have resulted from a complex interplay of biochemical events. Initially, the decreased protein levels observed in the tissues was traced to the leaking of the cell consequent to cell disruption or necrosis; these protein molecules ought to leak into the blood and thereby increasing the protein level of the serum[8]. Prior administration of each of the extracts and subsequent treatment of the rabbits with DNPH administration resulted in increased soluble protein levels when compared with DNPH-treated group, in all the tissues investigated. This finding agrees with the report of Lin and Wang[11]. Anthocyanin-rich treated groups showed the greater potential to maintain the basal level of protein concentration in the three tissues thereby exhibiting tissue regenerative potencies and tissue protein synthesis[20].
5. Conclusions
- This work shows that extracts from the calyces of Hibiscus sabdariffa contain bioactive principles which possess potent anti-cytotoxic properties. The mechanism by which the extracts of Hibiscus sabdariffa blocks tissue depletion of glucose and protein is not clear at this stage. However, it is likely that the extracts protected the tissues from damage by DNPH-induced free radical formation. The protection may also be due to impaired free radical propagation and / or complementation of the antioxidant defense system. Evidently, the aqueous extract of H. sabdariffa consumed as local beverage in parts of the world especially in West African sub-region has potent biological antioxidant properties against 2,4-DNPH-induced tissue damage. Its continued consumption after complete toxicological investigation may be encouraged on account of its natural antioxidant bioactivity.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML