-
Paper Information
- Next Paper
- Previous Paper
- Paper Submission
-
Journal Information
- About This Journal
- Editorial Board
- Current Issue
- Archive
- Author Guidelines
- Contact Us
Frontiers in Science
p-ISSN: 2166-6083 e-ISSN: 2166-6113
2012; 2(6): 214-220
doi: 10.5923/j.fs.20120206.14
Antimicrobial Activity of Cymbopogon Citratus (Lemon Grass) and It’s Phytochemical Properties
Ewansiha J. U.1, Garba S. A.1, Mawak J. D.2, Oyewole O. A.1
1Department of Microbiology, Federal University of Technology, Minna, Niger State, Nigeria
2Department of Microbiology, University of Jos, Plateau State, Nigeria
Correspondence to: Ewansiha J. U., Department of Microbiology, Federal University of Technology, Minna, Niger State, Nigeria.
| Email: |  |
Copyright © 2012 Scientific & Academic Publishing. All Rights Reserved.
The cold maceration and agar diffusion technique were employed to assess phytochemical properties and the antimicrobial potency of Cymbopogon citratus (lemongrass) against selected microbial pathogens using hexane, chloroform and methanol as extracting solvents. The mean zones of inhibition of the chloroform leaf and corresponding root extracts for the test organisms were Staphylococus aureus (11.33±1.15,11.66±2.52), Salmonella typhi (11.33±1.53,13.66±0.58), Escherichia coli (16.33±0.58,15.66±2.31) and Candida albicans (7.66±0.58,8.66±1.53) respectively. Hexane and methanol extracts showed no activity against the test organisms. The minimum inhibitory concentration (MIC) and the corresponding minimum bactericidal concentration (MBC) for chloroform leaf and root extracts were : Staphylococus aureus (24µg/ml, 28µg/ml), Salmonella typhi (20µg/ml, 28µg/ml), Escherichia coli (14µg/ml, 16µg/ml), Candida albicans (32µg/ml, 38µg/ml) and Staphylococus aureus (20µg/ml, 26µg/ml), Salmonella typhi (18µg/ml, 24µg/ml), Escherichia coli (14µg/ml, 16µg/ml), Candida albicans (28µg/ml, 32µg/ml) respectively. Phytochemical screening on Cymbopogon citratus showed that five active ingredients: Tannins, Flavonoids, Phenols, Carbohydrates and volatile oil were present in both the root and leaf parts. The mean zones of inhibition showed that Cymbopogon citratus exhibited an intermediate antimicrobial activity against the bacteria species while C. albicans was resistant. Higher dose of C. citratus may be recommended to exert a remarkable antimicrobial activity against the test organisms.
Keywords: Cold Maceration, Antimicrobial Potency, Minimum Inhibitory Concentration, Minimum Bactericidal Concentration, Phytochemical Screening
Cite this paper: Ewansiha J. U., Garba S. A., Mawak J. D., Oyewole O. A., "Antimicrobial Activity of Cymbopogon Citratus (Lemon Grass) and It’s Phytochemical Properties", Frontiers in Science, Vol. 2 No. 6, 2012, pp. 214-220. doi: 10.5923/j.fs.20120206.14.
Article Outline
1. Introduction
- Nature has been a source of medicinal agents for thousands of years and since the beginning of man. In Nigeria, almost all plants are medicinal and the application of medicinal plants especially in traditional medicine is currently well acknowledged and established as a viable profession[1]. In addition to providing the animal kingdom it's food, fuel and shelter, plants accumulate other phytochemical constituents - the secondary metabolites which are produced as by-product and are not directly useful to them. These secondary metabolites gives plants their medicinal value some of which include Alkaloids, Tannins, Saponins, Flavonoids, Antraquinones, Glycosides, Volatile oils, Terpenes, Essential oils, Resins[2].Medicinal plants have therefore been described as one in which one or more of its organs contain substances that can be used for therapeutic purpose[19]. It may be in the form of vegetable drugs which may either be organized (material which posses a cellular structure e.g. Leaf, bark petal,flower, stem, root, etc) or unorganized drugs (a cellular structural medicinal agents such as gums, balsams and Latex), such plants materials may be utilized in the form of decoctions in cold water or warm water, concoctions, preparations of soups, drinks etc made fully from many ingredients. They can also be used as infusions often made by pouring water on a specified plant material and allowing the mixture to stand for about 15 minutes[18]. For the past two decades, there has been an increasing interest in the investigation of different extracts obtained from traditional medicinal plants as potential sources of new antimicrobial agent[1]. Although it has been estimated that about one in four of all prescribed drugs' and almost 7,000 different medicaments contain compounds of plant origin or their derivatives with their commercial value being put at about $40 billion annually[5]. Indicated that about 33% of drugs produced in the developed countries are derived from plants[6]. Cymbopogon citratus of the Poaceae family is a tall, monocotyledonous aromatic perennial plant with slender sharp-edged green leaves, pointed apex that is native to tropical Asia. C. citratus is known as Guatemala in West Indian, or Madagascar lemongrass[9]. C. citratus is cultivated in Africa, the West Indies, Central and South America, and tropical regions. The linear leaves can grow up to 90 cm in height and 5 mm in width[9]. The aim and objectives of this work is to determine therapeutic potentials of the plant extract on some pathogenic microorganisms and the presence/variations of active principle in the plant parts.
2. Materials and Methods
2.1. Identification, Collection and Processing of Plant Materials
- The leaves and roots of Cymbopogon Citratus (Lemon grass) were collected from Bosso Low cost Area of Minna, Niger State Nigeria. It was identified by Dr. John Apah of the Department of Plant Development and Research, National Institute of Pharmaceutical Research and Development (NIPRD) Abuja. The plant samples were crushed and blended into smaller pieces to enhance the penetration of the extracting solvents into the plant cells, thus facilitating the release of the active principles.The cold maceration method as described by[25] was used. Two hundred grams (200g) of powdered Cymbopogon citratus samples (leaf and root) were weighed using a weighing balance into two 1000ml capacity conical flask. One litre each of the solvents (Hexane, Chloroform and Methanol) was added to each of the samples respectively. The conical flasks containing the mixtures were placed on a shaker for 24 hours. After 24 hours of shacking and mixing, it was next filtered using muslin cloth. The filtrates were then filtered again using suction pressure with the aid of a vacuum pump. The filtered extracts were concentrated using the rotary evaporator equipment after which they were dried on an evaporating dish at a temperature of 50℃ to 60℃ to a semi-solid form. A sticky semi-solid greenish substance was obtained for both samples. The extracts were stored in a well corked universal bottle.
2.2. Phytochemical Screening of Extracts
- Phytochemical analysis was performed to screen the extracts for the presence of the following active principles: Tannins, Flavonoids, Volatile oils, Phenol, Carbohydrates. All procedures were as described by Sofowora (1984).
2.2.1. Test for Tannins
- Zero point five milliliters (0.5mls) of each extract was added to 10.omls of distilled water and mixed with few drops of Ferric Chloride (FeCl3) solution. An immediate visible green precipitate is indicative of a positive test[23].
2.2.2. Test for Flavonoids
- One point zero milliliters (1.0mls) of each extract were dissolved in sodium hydroxide (NaOH) solution. The appearance of yellow solution, which disappeared on addition of HCL, indicates the presence of flavonoids[22] .
2.2.3. Test for Volatile Oils
- Volatile oils are characterized by their odour, oil-like appearance and ability to volatilize at room temperature. The plant materials were distilled with water by steam distillation and the distilates were collected in a graduated tube. The aqueous portion which separates automatically was returned to the distillation flask. The formation of emulsion which floats on top of the aqueous phase owing to its low density is indicative of the presence of volatile oils[21].
2.2.4. Test for Carbohydrates
- Three grams of powdered samples each of Cymbopogon citratus leaf and root were boiled separately in 50ml of distilled water on a water bath for 3 minutes. The mixtures were filtered while hot and the resulting filtrate allowed to cool. A few drops of Molisch’s reagent was added to 2ml of the leaf extract, a small quantity of concentrated sulphuric acid was added and allowed to form a lower layer. The procedure was repeated with the root extract. A purple ring at the interface of the liquids indicated the presence of carbohydrates. The mixtures were then shaken allowed to stand for 2 minutes and then diluted with 5ml of water. A purple precipitate also indicated the presence of carbohydrate[21] .
2.3. Preparation of Test Organisms
- The microorganisms used namely Staphylocuccus aureus, Escherichia coli, Salmonella typhi, Candida albicans were obtained from stock cultures in the Microbiology Laboratory of the National Institute for Pharmaceutical Research and Development (NIPRD), Idu Abuja,Nigeria. They were subcultured and identified based on their colonial morphology, microscopic appearance and specific biochemical reactions. The test organisms were sub cultured in 10ml broth each and incubated at 37℃ for 18 to 24 hours. After 24 hours, the organisms were sub cultured into a fresh Mueller Hinton Broth and incubated for 3hours which was used for all analyses.
2.4. Preparation of Extracts
- Zero point one grams (0.1g) of the hexane, chloroform and methanol extracts were weighed and dissolved in 5ml each of sterile distilled water. This gives 20mg/ml concentration each.
2.5. Antibacterial Assay of Crude Extracts
- Mueller Hinton Agar media was streaked uniformly according to the number of test organisms and was labelled appropriately; sterile cup borer (6mm) was used to bore holes in the culture media. The base of each wells were sealed with a drop of molten agar to prevent unwanted spread of the extracts. In a drop-wise manner, 1ml of the prepared extracts was added into each well and the cultures were allowed to stand for 30 minutes before they were transferred in to the incubator. The cultures were incubated for 24 -48 hours at 37℃ before final readings were taken. Control plates were also prepared for each test organisms without the addition of extracts. Zones of inhibition were measured to the nearest millimetre[7] .
2.6. Antifungal Assay of Crude Extracts
- The agar diffusion method[4] was employed. The test organism Candida albicans was inoculated into test tubes containing sabouraud dextrose broth and incubated at room temperature for 72 hours. The organisms were subcultured into sabouraud dextrose agar by the pour plate method. A sterile cork borer (6mm) was used to bore holes in the culture media and the base of the wells was sealed with a drop of molten agar to prevent unwanted spreading of the extracts. In a drop-wise manner, 1ml of the extract was added into each of the well and the cultures were allowed to stand for 30 minutes before incubation at room temperature for 48 hours. After 48 hours of active growth, the zones of inhibition were measured with the aid of a meter rule considering the diameter of the cork borer. A control plate was also prepared for each test organism without the addition of extracts.
2.7. Column chromatography
- The micro scale flash column chromatographic method according to[10] was used to separate the fractions of the chloroform extracts. The column was prepared by plugging a Pasteur pipette with a small amount of cotton using a simple dry-pack method and with a wood applicator stick it was tamped down lightly. One hundred milligram silica gel was added as the stationary phase. The column was next pre-eluted by the addition of solvent (Chloroform) and it was allowed to flow slowly down the column by gravity and the flow was also slightly aided by the application of air at the top of the column with the aid of a pipette bulb. The column was loaded with the sample by the wet method which involves dissolving 100mg of the extract in the solvent before addition. It was then eluted as necessary by forcing more solvent through the column with the aid of a Pasteur pipette bulb which also prevents the silica gel from going dry. The fractions were collected in test tubes according to their colour development.
2.8. Antimicrobial Analysis of C.citratus Fractions
- The same method for the determination of the antimicrobial activity of the crude extracts against the test organisms (both for the fungi and bacteria species) was used for the fractions obtained from the column chromatography. The only difference is that in this case the fractions were used in place of the crude extract and each culture was incubated appropriately based on the species under test.
2.9. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
2.9.1. Preparation of Extract
- Zero point one gram (0.1g) of extract was dissolved in 100ml w/v of distilled water to give 100mg/100ml which is equivalent to 1000 µg/ml. From the solution above, 5ml was diluted with 50ml v/v of distilled water from which the desired concentrations such as 2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22, 24, 26, 28, 30, 32, 34, 36, 38 and 40ug/ml were prepared by diluting each desired concentration in 100ml v/v of distilled water from the above 50ml.
2.9.2. Dilution Susceptibility Test
- The dilution susceptibility tests method was used to determine the minimum inhibitory concentration and the minimum bactericidal concentration of Cymbopogon citratus root and leaf extracts. Series of test tubes containing Mueller-Hinton broth and Sabouraud Dextrose broth respectively were prepared. Different concentration of crude chloroform extracts of Cymbopogon citratus root and leaf ranges from 2µg/ml, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22, 24, 26, 28, 30, 32, 34, 36, 38 and 40µg/ml were prepared and 1ml of each of this concentrations of the extract were mixed with the prepared media. Serial dilutions of overnight cultures of the organisms were made and each dilution compared to a McFarland tube (0.5) equivalent to 1×108cfu/ml. The inoculated broths were incubated at 37℃ and 25-30℃for bacteria and fungi respectively for 24 hours. After 24 hours, the tubes were observed for growth and recorded as the minimum inhibitory concentration (MIC). The tubes with no growth after 24 hours were sub-cultured on freshly prepared Mueller – Hinton agar and sabouraud dextrose agar by the streaking method for bacteria and fungi respectively. The culture media were incubated appropriately for 24 hours and then observed for growth. After 24 hours, the lowest concentration from which the microorganisms did not recover and grow when transferred to the fresh media was recorded as the minimum bactericidal concentration (MBC)[7].
2.10. Standard Antibiotic Susceptibility Test
- Disc diffusion method was used in this test as described by[24]. Six millimetre (6mm) commercially prepared antibiotic paper discs were used. The antibiotic discs used and their concentrations are Ciprofloxacin (30µg), Erythromycin (15µg), Tetracycline (30µg) and Ketoconazole (15µg) for Staphylococcus aureus, Salmonella typhi, Escherichia coli and Candida albicans respectively. The discs were applied in accordance to the National committee for clinical laboratory standard. The antibiotics used in the test were chosen after a preliminary survey and to reflect the range of drugs commonly prescribed for the treatment caused by the test organisms
2.11. Susceptibility Test
- Sterile swab sticks were used to transfer the test organisms into tubes containing physiological normal saline to form a suspension. Prepared Mueller Hinton Agar and sabouraud dextrose agar were inoculcated appropriately with the test organisms (Staphylococus aureus, Salmonella typhi, Escherichia coli and candida albicans) by dipping the sterile swab sticks into the suspension and removing excess inoculum by pressing and rotating the swab firmly against the side of the tube. The inoculums were streaked all over the surface of the medium rotating the plates through an angle of 60° after each application [24]. The inoculated plates were allowed to dry for a few minutes at room temperature with the lid closed. The antibiotic discs were then placed ascetically on the inoculated plates using a pair of sterile forceps. Each disc was gently pressed down to ensure even contact with the medium. The plates were incubated at 37℃ for 24 hours in the case of bacteria while that of fungi were incubated at room temperature for 48 hours. At the end of the incubation period, the results were recorded as sensitive or resistance based on the occurrence of zone of inhibition respectively.
3. Results
3.1. Antimicrobial Assay of Crude Extracts of C.citratus
- The mean zones of inhibition of the leaf and root extracts of Cymbopogon Citratus against Staphylococus aureus, Salmonella typhi, Eschenchia coli and Caudida albicans are shown in Table 3.1. The results in table 1 showed the spread of data around the mean of each replicates of the zones of inhibition. Staphylococcus aureus, Salmonella typhi and Escherichia coli showed an intermediate susceptibility (11.33±1.15 to 16.33±0.58) for leaf extract and (11.66±2.52 to 15.66±2.31) for root extract while Candida albicans was resistant with 7.66±0.58 for the leaf extract and 8.66 ±1.53 for the root extract.
3.2. Mean Zones of Inhibition of Crude Extracts of C.citratus with Standard Antibiotics
- Table 3.2 showed the results of the comparison of the zones of inhibition caused by the extracts of C. citratus with standard antibiotics. The bacteria species showed an intermediate susceptibility with mean zones of inhibition of 11.33±1.15 to 16.33±0.58 to the extract while showing susceptibility to the standard antibiotics with mean zones of inhibition of 15±1.15 to 19.33±0.58. Table 3.2 also showed that Candida albicans was markedly resistant with mean zones of inhibition of 7.66±0.58 and 8.66 ±1.53 to the crude leaf and root extract respectively and susceptible to the standard antibiotic.
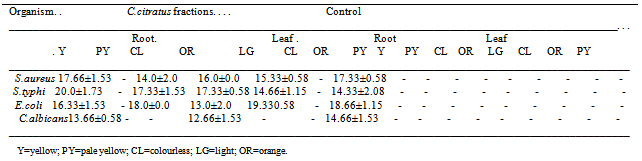
3.3. Antimicrobial Activity of Column Chromatography Fractions of C.citratus
- A total of eight (8) different fractions were eluted from the Chloroform extract of the leaf and root of C. citratus. Of the 8, 5 (yellow, pale yellow, colorless, orange and light green) were from the root extract while 3 (colourless, orange and pale yellow) were from the leaf extract (Table 3.3). The fractions showed higher antimicrobial activity than the crude extract against the organisms which indicates a pure form of the crude extract after passing through the column chromatography process (Table 3.3).
3.4. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of C.citratus Extracts
|
|
|
|
|
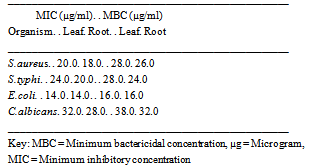
- The result of minimum inhibitory concentration (MIC) and Minimum bactericidal concentration (MBC) of chloroform extract of Cymbopogon citratus root is shown in Table 3.4. The minimum concentration of Cymbopogon citratus causing inhibition and total death of microorganism was 14.0µg/ml and 38 µg /ml respectively.
3.5. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of Chloroform Extract of Cymbopogon Citratus
- The result of minimum inhibitory concentration (MIC) and Minimum bactericidal concentration (MBC) of chloroform extract of Cymbopogon citratus root is shown in Table 3.5. The result showed that the minimum concentration of Cymbopogon citratus causing inhibition and the corresponding minimum concentration causing total death of microorganisms are leaf : 20.0µg /ml & 28.0µg/ml, 24.0µg/ml & 28.0µg/ml, 14.0µg/ml & 16.0µg/ml, 32.0µg/ml,38µg/ml; root : 18.0 µg /ml & 26.0 µg /ml, 20.0 µg /ml & 24.0 µg /ml, 14.0 µg /ml & 16.0 µg /ml, 28.0 µg /ml & 32.0 µg /ml for Staphylococus aureus, Salmonella typhi, Eschenchia coli and Caudida albicans respectively.
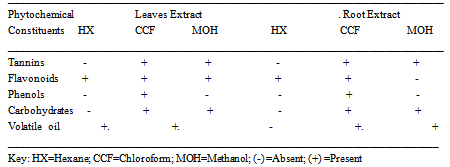
3.6. Phytochemical Analysis
- The result of phytochemical screening on Cymbopogon Citratus showed that five active ingredients were present in both the root and leaf parts. These include Tannins, Flavonoids, Phenols, Carbohydrates and volatile oil (Table 3.6). Phenol is only present in the chloroform extract and absent in both hexane and methanol extract. Also chloroform extract had all five active ingredients identified while hexane had 2 each for both leaf and root extract and methanol had 3 for leaf extract and 2 for root extract (Table 3.6).
4. Discussion
- Extraction and Phytochemical screening of bioactive agents from medicinal plants permits the demonstration of their physiological activities. The phytochemical analyses showed that Flavonoids and Volatile oil are present in hexane extract; Tannins, Flavonoids, Phenol, Carbohydrates and Volatile oil were present in chloroform extract; Tanins, Flavonoids and Carbohydrates were present in methanol leaf extract while only Tannins and Carbohydrates were present in methanol root extract (Table 5). Phytochemical screening of Cymbopogon citratus also revealed the presence of volatile oil, also call essential oil and according to[11], the presence of volatile oil gives plant their specific aromas which is confirmed by the aroma produced by this plant and are extracted by solvent Extraction. The presence of volatile oil also confirms the report of[20] of the application of Cymbopogon citrarus in perfumery, cosmetics and soap industry.According to[12], tannins and phenolic compounds have been found to inhibit bacterial and fungal growth and also capable of protecting certain plants against infection. According to the report of[18], that phytochemical component has antifungal properties which were confirmed in this study. The presence of tannins in the plant extract agrees with the report of[11] that tannins are important in herbal medicine and they are applied in arresting bleeding and wound healing. Tannins and tannic acid own their stringent action to the fact that they precipitate protein and render them resistant to attack by proteolytic enzymes, internally; they form a pellicle of coagulated protein over the lining of the alimentary tract. The antifungal activity against Caudida albicans is low with mean zone of inhibition of 7.66±0.58 and 8.66 ±1.53 with an MIC of 28µg/ml and 32µg/ml for leaf and root extract respectively which suggest that the use of this plant for therapeutic purpose against infections of Caudida albicans may not be fully successful as the fungi may show resistance to the plant.This in no doubt confirm partly the report that Cymbopogon citratus has been used against gastrointestinal disturbances[16] but might require high dosage due to the level of antimicrobial activity it showed in this research result. The root of Cymbopogon citratus showed more antimicrobial activity than the leaf extracts (Table 1) despite the presence of bioactive agents in both, which showed that there are more different types of active ingredient in different plant species and different plant parts even though they have the same generic name i.e. the type of tannins present in one plant or plant part may be different from the tannins present in another plant species or plant part[11]. Tannins have also been reported to have antidiarrheal, homeostatic and antihemorrhagal activity,[3]. According to[14] there are three different types of tannins; Hydrolysable tannins, Non-Hydrolysable tannins or condensed tannins and Pseudo tannins. Various volatile oils in plant have been reported to have medicinal values ranging from skin treatment to remedy for cancer[13]. The isolation of volatile oils in Cymbopogon citratus confirms the activity showed against the test organisms by this plant and also in part confirms the report of[4] of the oils isolated from same plant by distillation to exhibit great antibacterial activity and also confirms the potency of this particular plant against skin cancer prevention as reported by[16]. In a 13-oil studies, lemongrass oil was found to be among the most active against human dermatophyte strains inhibiting 80% of strains as reported by[15] and[3], this is confirmed by the antifungal activity of Cymbopogon Citratus against strains of fungi species used as test organism and also confirm reports by traditional users of lemongrass against ring worm infections. According to the phytochemical result, phenol was not found in both hexane and methanol extract but only in chloroform extract. This may contribute to the high antimicrobial activity showed by the chloroform extract which is absent in the other two solvents extracts. Methanol extracts shows no antimicrobial activity which is in agreement with the report of[3] of extracts of ethanol which have almost same properties with methanol used in this research work on the same plant (Cymbopogon citratus) having no antimicrobial activity on the test organisms. The inhibitory activities of the plant extract on the test organisms indicate that the plant possess active ingredients which may be chloroform soluble. The column chromatography of Cymbopogon citratus chloroform extract revealed 8 different fractions, 5[FR1 (yellow), FR2 (pale yellow), FR3 (colorless), FR4 (orange), FR5 (light green) for the root and 3 (FL1 (colourless), FL2 (orange), FL3 (pale yellow) for the leaf (Table 3). The fractions had antimicrobial activity higher than the crude extracts of same plant. This may be as a result of the fact that the chromatography procedure purified the extract. On the other hand the crude extract may still contain some impurities which may likely prevent their activity. Therefore purification is very important because the presence of some ingredients which reduce the activity of the bioactive components is eliminated, concentrating the active component hence, increasing the activity of the active components..
5. Conclusions
- The results of this study on Cymbopogon citratus have led to the following conclusions:The extracts of Cymbopogon citratus leaf and roots (chloroform extracts) possessed intermediate antimicrobial activity against Staphylococcus aureus, Salmonella typhi and E. coli. Candida albicans was markedly resistant to lemongrass. Lemon grass has low antimicrobial activity on the test pathogens as compared to Tetracycline, Chlorophenicol, Erythromycin and ketoconazone. The MIC of Cymbopogon citratus reveals that a higher dose of the plant extract is required to bring about a significant activity in the body.Five active ingredients were identified in the plant (root and leaf) which include flavonoids, tannins, phenol, volatile oil and carbohydrates.
 Abstract
Abstract Reference
Reference Full-Text PDF
Full-Text PDF Full-Text HTML
Full-Text HTML